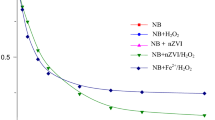
Abstract
The effect of pH, temperature, zero-valent iron (ZVI) dosage and reaction duration on nitrobenzene (NB) degradation by micro-sized ZVI was investigated in batch experiments and the electron efficiency (EE) was evaluated at constant pH. The experimental results showed that the reaction rate constant attained 0.616 min−1, which was far higher than reported in previous studies. The decrease in pH and increase in temperature of the reaction bath promoted the reaction kinetics. ZVI dosage and reaction duration were two key factors affecting NB reduction and EE. A lower ZVI dosage and a slightly longer reaction duration could afford an optimised EE and optimum NB removal. A prolonged reaction or too high a ZVI dosage resulted in increased ZVI waste.
Similar content being viewed by others
References
Agrawal, A., & Tratnyek, P. G. (1995). Reduction of nitro aromatic compounds by zero-valent iron metal. Environmental Science Technology, 30, 153–160. DOI: 10.1021/es950211h.
American Water Works Association, American Public Health Association, Water Environment Federation (2005). Standard methods for the examination of water and wastewater (21th ed.). Washington, D.C., USA: American Water Works Association/American Public Health Association/Water Environment Federation.
Anotai, J., Sakulkittimasak, P., Boonrattanakij, N., & Lu, M. C. (2009). Kinetics of nitrobenzene oxidation and iron crystallization in fluidized-bed Fenton process. Journal of Hazardous Materials, 165, 874–880. DOI: 10.1016/j.jhazmat.2008.10.062.
Arnold, W. A., & Roberts, A. L. (2000a). Inter- and intraspecies competitive effects in reactions of chlorinated ethylenes with zero-valent iron in column reactors. Environmental Engineering Science, 17, 291–302. DOI: 10.1089/ees.2000.17.291.
Arnold, W. A., & Roberts, A. L. (2000b). Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles. Environmental Science & Technology, 34, 1794–1805. DOI: 10.1021/es990884q.
Bell, L. S., Devlin, J. F., Gillham, R. W., & Binning, P. J. (2003). A sequential zero valent iron and aerobic biodegradation treatment system for nitrobenzene. Journal of Contaminant Hydrology, 66, 201–217. DOI: 10.1016/s0169-7722(03)00035-4.
Devlin, J. F., Klausen, J., & Schwarzenbach, R. P. (1998). Kinetics of nitroaromatic reduction on granular iron in recirculating batch experiments. Environmental Science & Technology, 32, 1941–1947. DOI: 10.1021/es970896g.
Devlin, J. F., & Allin, K. O. (2005). Major anion effects on the kinetics and reactivity of granular iron in glass-encased magnet batch reactor experiments. Environmental Science & Technology, 39, 1868–1874. DOI: 10.1021/es040413q.
Dong, J., Zhao, Y. S., Zhao, R., & Zhou, R. (2010). Effects of pH and particle size on kinetics of nitrobenzene reduction by zero-valent iron. Journal of Environmental Science, 22, 1741–1747. DOI: 10.1016/s1001-0742(09)60314-4.
He, S. L., Wang, L. P., Zhang, J., & Hou, M. F. (2009). Fenton pre-treatment of wastewater containing nitrobenzene using ORP for indicating the endpoint of reaction. Procedia Earth and Planetary Science, 1, 1268–1274. DOI: 10.1016/j.proeps.2009.09.196.
Huang, Y. H., Zhang, T. C., Shea, P. J., & Comfort, S. D. (2003). Effects of oxide coating and selected cations on nitrate reduction by iron metal. Journal of Environmental Quality, 32, 1306–1315. DOI: 10.2134/jeq2003.1306.
Huang, Y. H., & Zhang, T. C. (2006). Reduction of nitrobenzene and formation of corrosion coatings in zerovalent iron systems. Water Research, 40, 3075–3082. DOI: 10.1016/j.watres.2006.06.016.
Hung, H. M., Ling, F. H., & Hoffmann, M. R. (2000). Kinetics and mechanism of the enhanced reductive degradation of nitrobenzene by elemental iron in the presence of ultrasound. Environmental Science & Technology, 34, 1758–1763. DOI: 10.1021/es990385p.
Johnson, T. L., Fish, W., Gorby, Y. A., & Tranyek, P. G. (1998). Degradation of carbon tetrachloride by iron metal: Complexation effects on the oxide surface. Journal of Contaminant Hydrology, 29, 379–398. DOI: 10.1016/s0169-7722(97)00063-6.
King, D. W., Lounsbury, H. A., & Millero, F. J. (1995). Rates and mechanism of Fe(II) oxidation at nanomolar total iron concentrations. Environmental Science & Technology, 29, 818–824. DOI: 10.1021/es00003a033.
Klausen, J., Ranke, J., & Schwarzenbach, R. P. (2001). Influence of solution composition and column aging on the reduction of nitroaromatic compounds by zero-valent iron. Chemosphere, 44, 511–517. DOI: 10.1016/s0045-6535(00)00385-4.
Kuşçu, Ö. S., & Sponza, D. T. (2009). Effect of increasing nitrobenzene loading rates on the performance of anaerobic migrating blanket reactor and sequential anaerobic migrating blanket reactor/completely stirred tank reactor system. Journal of Hazardous Materials, 168, 390–399. DOI: 10.1016/j.jhazmat.2009.02.060.
Lavine, B. K., Auslander, G., & Ritter, J. (2001). Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchemical Journal, 70, 69–83. DOI: 10.1016/s0026-265x(01)00075-3.
Liou, Y. H., Lo, S. L., Lin, C. J., Hu, C. Y., Kuan, W. H., & Weng, S. C. (2005). Methods for accelerating nitrate reduction using zerovalent iron at near-neutral pH: Effects of H2-reducing pretreatment and copper deposition, Environmental Science & Technology, 39, 9643–9648. DOI: 10.1021/es048038p.
Liu, H., Wang, Q., Wang, C., & Li, X. Z. (2013). Electron efficiency of zero-valent iron for groundwater remediation and wastewater treatment. Chemical Engineering Journal, 215–216, 90–95. DOI: 10.1016/j.cej.2012.11.010.
Mantha, R., Taylor, K. E., Biswas, N., & Bewtra, J. K. (2001). A continuous system for Fe0 reduction of nitrobenzene in synthetic wastewater. Environmental Science & Technology, 35, 3231–3236. DOI: 10.1021/es0014943.
Mu, Y., Yu, H. Q., Zheng, J. C., Zhang, S. J., & Sheng, G. P. (2004). Reductive degradation of nitrobenzene in aqueous solution by zero-valent iron. Chemosphere, 54, 789–794. DOI: 10.1016/j.chemosphere.2003.10.023.
Noubactep, C. (2009). An analysis of the evolution of reactive species in Fe0/H2O systems. Journal of Hazardous Materials, 168, 1626–1631. DOI: 10.1016/j.jhazmat.2009.02.143.
Park, B., & Dempsey, B. A. (2005). Heterogeneous oxidation of Fe(II) on ferric oxide at neutral pH and a low partial pressure of O2. Environmental Science Technology, 39, 6494–6500. DOI: 10.1021/es0501058.
Ritter, K., Odziemkowski, M. S., Simpgraga, R., Gillham, R. W., & Irish, D. E. (2003). An in situ study of the effect of nitrate on the reduction of trichloroethylene by granular iron. Journal of Contaminant Hydrology, 65, 121–136. DOI: 10.1016/s0169-7722(02)00234-6.
Scherer, M. M., Johnson, K. M., Westall, J. C., & Tratnyek, P. G. (2001). Mass transport effects on the kinetics of nitrobenzene reduction by iron metal. Environmental Science & Technology, 35, 2804–2811. DOI: 10.1021/es0016856.
Tamaura, Y., Ito, K., & Katsura, T. (1983). Transformation of γ-FeO(OH) to Fe3O4 by adsorption of iron (II) ion on γ-FeO(OH). Journal of the Chemical Society, Dalton Transactions, 1983, 189–194. DOI: 10.1039/dt9830000189.
Walter, W. G. (1926). Corrosion of iron. Chemical Reviews, 2, 419–435. DOI: 10.1021/cr60008a003.
Wüst, W. F., Köber, R., Schlicker, O., & Dahmke, A. (1999). Combined zero-and first-order kinetic model of the degradation of TCE and cis-DCE with commercial iron. Environmental Science & Technology, 33, 4304–4309 DOI: 10.1021/es980439f.
Yin, W. Z., Wu, J. H., Li, P., Wang, X. D., Zhu, N. W., Wu, P. X., & Yang, B. (2012). Experimental study of zero-valent iron induced nitrobenzene reduction in groundwater: The effects of pH, iron dosage, oxygen and common dissolved anions. Chemical Engineering Journal, 184, 198–204. DOI: 10.1016/j.cej.2012.01.030.
Zhao, L., Ma, J., Sun, Z. Z., & Liu, H. (2009). Influencing mechanism of temperature on the degradation of nitrobenzene in aqueous solution by ceramic honeycomb catalytic ozonation. Journal of Hazardous Materials, 167, 1119–1125. DOI: 10.1016/j.jhazmat.2009.01.116.
Zhu, C. Z., Ouyang, B., Wang, J. Q., Huang, L., Dong, W. B., & Hou, H. Q. (2007). Photochemistry in the mixed aqueous solution of nitrobenzene and nitrous acid as initiated by the 355 nm UV light. Chemosphere, 67, 855–861. DOI: 10.1016/j.chemosphere.2006.11.031.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, CQ., Fu, DT. & Chen, X. Nitrobenzene degradation by micro-sized iron and electron efficiency evaluation. Chem. Pap. 68, 1350–1357 (2014). https://doi.org/10.2478/s11696-014-0573-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-014-0573-3