Abstract
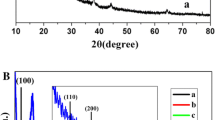
The effects of Ag-doping on the physico-chemical, spectral, surface, and catalytic properties of the FeMgO system with various Fe2O3 loadings were investigated. The dopant (Ag) molar ratio varied between 0.01 % and 0.05 %. The techniques employed for characterisation of catalysts were TG/DTG, XRD, ESR, N2 adsorption at −196°C, and catalytic decomposition of H2O2 at 25–35°C. The results obtained revealed that the investigated catalysts consisted of nanosized MgO as the major phase, apart from the MgFe2O4 and/or Fe3O4 phases. ESR result of the FeMgO system revealed the presence of paramagnetic species as a result of Ag-doping. The textural properties including SBET, porosity and St were modified by Ag-doping. The doping process with Ag-species improved the catalytic activity of the FeMgO system. Increasing the calcination temperature from 400°C to 800°C increased the catalytic activity (k*30 °C) of 0.05 AgFeMgO in H2O2 decomposition by 21.2 times.
Similar content being viewed by others
References
Al-Sayari, S., Carley, A. F., Taylor, S. H., & Hutchings, G. J. (2007). Au/ZnO and Au/Fe2O3 catalysts for CO oxidation at ambient temperature: comments on the effect of synthesis conditions on the preparation of high activity catalysts prepared by coprecipitation. Topics in Catalysis, 44, 123–128. DOI: 10.1007/s11244-007-0285-9.
Anand, S., & Srivastava, O. N. (2002). Effect of AgNO3 additive/ doping on microstructure and superconductivity of the Pb doped Hg: 1223 thin film prepared through spray pyrolysis. Journal of Physics and Chemistry of Solids, 63, 1647–1653. DOI: 10.1016/s0022-3697(01)00249-9.
Bachari, K., Touileb, A., Saadi, A., Halliche, D., & Cherifi, O. (2010). Synthesis and structural characterization of iron-modified folded sheet mesoporous materials. Journal of Porous Materials, 17, 573–581. DOI: 10.1007/s10934-009-9326-z.
Bandara, J., Mielczarski, J. A., Lopez, A., & Kiwi, J. (2001). Sensitized degradation of chlorophenols on iron oxides induced by visible light: Comparison with titanium oxide. Applied Catalysis B: Environmental, 34, 321–333. DOI: 10.1016/s0926-3373(01)00225-9.
Berlier, G., Spoto, G., Bordiga, S., Ricchiardi, G., Fisicaro, P., Zecchina, A., Rossetti, I., Selli, E., Forni, L., Giamello, E., & Lamberti, C. (2002). Evolution of extraframework iron species in Fe silicalite: 1. Effect of Fe content, activation temperature, and interaction with redox agents. Journal of Catalysis, 208, 64–82. DOI: 10.1006/jcat.2002.3535.
Boudjemaa, A., Boumaza, S., Trari, M., Bouarab, R., & Bouguelia, A. (2009). Physical and photo-electrochemical characterizations of α-Fe2O3. Application for hydrogen production. International Journal of Hydrogen Energy, 34, 4268–4274. DOI: 10.1016/j.ijhydene.2009.03.044.
Brinen, J. S., Schmitt, J. L., Doughman, W. R., Achorn, P. J., Siegel, L. A., & Delgass, W. N. (1975). X-ray photoelectron spectroscopy studies of the rhodium on charcoal catalyst: II. Dispersion as a function of reduction. Journal of Catalysis, 40, 295–300. DOI: 10.1016/0021-9517(75)90259-6.
Brückner, A., Lück, R., Wieker, W., Fahlke, B., & Mehner, H. (1992). E.p.r. study on the incorporation of Fe(III) ions in ZSM-5 zeolites in dependence on the preparation conditions. Zeolites, 12, 380–385. DOI: 10.1016/0144-2449(92)90034-m.
Brunauer, S., Emmett, P. H., & Teller, E. (1938). Adsorption of gases in multimolecular layers. Journal of the American Chemical Society, 60, 309–319. DOI: 10.1021/ja01269a023.
Cao, J. L., Wang, Y., Yu, X. L., Wang, S. R., Wu, S. H., & Yuan, Z. Y. (2008). Mesoporous CuO-Fe2O3 composite catalysts for low-temperature carbon monoxide oxidation. Applied Catalysis B: Environmental, 79, 26–34. DOI: 10.1016/j.apcatb.2007.10.005.
Chen, H. L., Wu, L. G., & Zhu, C. L. (2000). PVA membrane filled β-cyclodextrin for separation of isomeric xylenes by pervaporation. Chemical Engineering Journal, 78, 159–164. DOI: 10.1016/s1385-8947(00)00154-6.
Choa, Y. H., Yang, J. K., Yang, W. J., & Auh, K. H. (2003). Synthesis and characterization of isolated iron oxide nanoparticle dispersed in MgO matrix. Journal of Magnetism and Magnetic Materials, 266, 20–27. DOI: 10.1016/s0304-8853(03)00451-7.
Costa, R. C. C., Lelis, M. F. F., Oliveira, L. C. A., Fabris, J. D., Ardisson, J. D., Rios, R. R. V. A., Silva, C. N., & Lago, R. M. (2006). Novel active heterogeneous Fenton system based on Fe3−x MxO4 (Fe, Co, Mn, Ni): The role of M2+ species on the reactivity towards H2O2 reactions. Journal of Hazardous Materials, 129, 171–178. DOI: 10.1016/j.jhazmat.2005.08.028.
Decyk, P., Trejda, M., Ziolek, M., Kujawa, J., Głaszczka, K., Bettahar, M., Monteverdi, S., & Mercy, M. (2003). Physicochemical and catalytic properties of iron-doped silica—the effect of preparation and pretreatment methods. Journal of Catalysis, 219, 146–155. DOI: 10.1016/s0021-9517(03)00186-6.
Deraz, N. A. M. (2001). Effect of Ag2O doping on surface and catalytic properties of cobalt-magnesia catalysts. Materials Letters, 51, 470–477. DOI: 10.1016/s0167-577x(01)00337-8.
Deraz, N. M. (2008). Production and characterization of pure and doped copper ferrite nanoparticles. Journal of Analytical and Applied Pyrolysis, 82, 212–222. DOI: 10.1016/j.jaap.2008.03.009.
Deraz, N. M. (2009). Characterization and catalytic performance of pure and Li2O-doped CuO/CeO2 catalysts. Applied Surface Science, 255, 3884–3890. DOI: 10.1016/j. apsusc.2008.10.068.
El-Molla, S. A. (2006). Dehydrogenation and condensation in catalytic conversion of iso-propanol over CuO/MgO system doped with Li2O and ZrO2. Applied Catalysis A: General, 298, 103–108. DOI: 10.1016/j.apcata.2005.09.029.
El-Shobaky, G. A., & Deraz, N. A. M. (2001). Surface and catalytic properties of cobaltic oxide supported on active magnesia. Materials Letters, 47, 231–240. DOI: 10.1016/s0167-577x(00)00240-8.
El-Shobaky, G. A., & Mostafa, A. A. (2003). Solid-solid interactions in Fe2O3/MgO system doped with aluminium and zinc oxides. Thermochimica Acta, 408, 75–84. DOI: 10.1016/s0040-6031(03)00323-x.
El-Shobaky, G. A., Shouman, M. A., & El-Khouly, S. M. (2003). Effect of silver oxide doping on surface and catalytic properties of Co3O4/Al2O3 system. Materials Letters, 58, 184–190. DOI: 10.1016/s0167-577x(03)00442-7.
Fuente, S. A., Belelli, P. G., Branda, M. M., Ferullo, R. M., & Castellani, N. J. (2009). Formation of Ag2, Au2 and AgAu particles on MgO(1 0 0): DFT study on the role of support-induced charge transfer in metal-metal interactions. Applied Surface Science, 255, 7380–7384. DOI: 10.1016/j.apsusc.2009.04.004.
Hamal, D. B., & Klabunde, K. J. (2007). Synthesis, characterization, and visible light activity of new nanoparticle photocatalysts based on silver, carbon, and sulfur-doped TiO2. Journal of Colloid and Interface Science, 311, 514–522. DOI: 10.1016/j.jcis.2007.03.001.
Ida, T., Tsuiki, H., Ueno, A., Tohji, K., Udagawa, Y., Iwai, K., & Sano, H. (1987). Characterization of iron oxide in Fe2O3/SiO2 catalyst. Journal of Catalysis, 106, 428–439. DOI: 10.1016/0021-9517(87)90255-7.
Khaleel, A., Shehadil, I., & Al-Shamisi, M. (2010). Nanostructured chromium-iron mixed oxides: Physicochemical properties and catalytic activity. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 355, 75–82. DOI: 10.1016/j.colsurfa.2009.11.041.
Kwan, W. P., & Voelker, B. M. (2003). Rates of hydroxyl radical generation and organic compound oxidation in mineralcatalyzed Fenton-like systems. Environmental Science & Technology, 37, 1150–1158. DOI: 10.1021/es020874g.
Lee, E. K., Jung, K. D., Joo, O. S., & Shul, Y. G. (2005). Influence of iron precursors on catalytic wet oxidation of H2S to sulfur over Fe/MgO catalysts. Journal of Molecular Catalysis A: Chemical, 239, 64–67. DOI: 10.1016/j.molcata.2005.05. 034.
Lin, D. H., Coudurier, G., & Vedrine, J. C. (1989). Fe-ZSM-5: Physicochemical and catalytic properties. Studies in Surface Science and Catalysis, 49, 1431–1448. DOI: 10.1016/s0167-2991(08)62028-5.
Lippens, B. C., & de Boer, J. H. (1965). Studies on pore system in catalysis: V. The t method. Journal of Catalysis, 4, 319–323. DOI: 10.1016/0021-9517(65)90307-6.
Liu, X., Shen, K., Wang, Y., Wang, Y., Guo, Y., Guo, Y., Yong, Z., & Lu, G. (2008). Preparation and catalytic properties of Pt supported Fe-Cr mixed oxide catalysts in the aqueousphase reforming of ethylene glycol. Catalysis Communications, 9, 2316–2318. DOI: 10.1016/j.catcom.2008.05.035.
Liu, Z., Hao, J., Fu, L., & Zhu, T. (2003). Study of Ag/La0.6 Ce0.4CoO3 catalysts for direct decomposition and reduction of nitrogen oxides with propene in the presence of oxygen. Applied Catalysis B: Environmental, 44, 355–370. DOI: 10.1016/s0926-3373(03)00103-6.
Meshkani, F., & Rezaei, M. (2010). Effect of process parameters on the synthesis of nanocrystalline magnesium oxide with high surface area and plate-like shape by surfactant assisted precipitation method. Powder Technology, 199, 144–148. DOI: 10.1016/j.powtec.2009.12.014.
Mishakov, I. V., Bedilo, A. F., Richards, R. M., Chesnokov, V. V., Volodin, A. M., Zaikovskii, V. I., Buyanov, R. A., & Klabunde, K. J. (2002). Nanocrystalline MgO as a dehydrohalogenation catalyst. Journal of Catalysis, 206, 40–48. DOI: 10.1006/jcat.2001.3474.
Mucka, V., & Tabacik, S. (1991). Catalytic properties of nickelcobalt mixed oxides and the influence of ionizing radiation. Radiation Physics and Chemistry, 38, 285–290. DOI: 10.1016/1359-0197(91)90094-I.
Parida, K. M., Dash, S. S., Mallik, S., & Das, J. (2005). Effect of heat treatment on the physico-chemical properties and catalytic activity of manganese nodules leached residue towards decomposition of hydrogen peroxide. Journal of Colloid and Interface Science, 290, 431–436. DOI: 10.1016/j.jcis.2005.04.056.
Radwan, N. R. E. (2006). Influence of La2O3 and ZrO2 as promoters on surface and catalytic properties of CuO/MgO system prepared by sol-gel method. Applied Catalysis A: General, 299, 103–121. DOI: 10.1016/j.apcata.2005.10.008.
Rao, G. R., Sahu, H. R., & Mishra, B. G. (2003). Surface and catalytic properties of Cu-Ce-O composite oxides prepared by combustion method. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 220, 261–269. DOI: 10.1016/s0927-7757(03)00080-3.
Renuka, N. K. (2010). A green approach for phenol synthesis over Fe3+/MgO catalysts using hydrogen peroxide. Journal of Molecular Catalysis A: Chemical, 316, 126–130. DOI: 10.1016/j.molcata.2009.10.010.
Sanli, A. E., & Aytaç, A. (2011). Response to Disselkamp: Direct peroxide/peroxide fuel cell as a novel type fuel cell. International Journal of Hydrogen Energy, 36, 869–875. DOI: 10.1016/j.ijhydene.2010.09.038.
Shaheen, W. M. (2002). Thermal solid-solid interaction and catalytic properties of CuO/Al2O3 system treated with ZnO and MoO3. Thermochimica Acta, 385, 105–116. DOI: 10.1016/s0040-6031(01)00710-9.
Shaheen, W. M. (2006). Thermal solid-solid interactions and catalytic properties of V2O5/Al2O3 system treated with Li2O and Ag2O. Materials Science and Engineering: B, 135, 30–37. DOI: 10.1016/j.mseb.2006.08.056.
Shaheen, W. M. (2008). Thermal solid-solid interactions and physicochemical properties of NiO/Fe2O3 system doped with K2O. Thermochimica Acta, 470, 18–26. DOI: 10.1016/j. tca.2008.01.012.
She, X., & Flytzani-Stephanopoulos, M. (2006). The role of Ag-O-Al species in silver-alumina catalysts for the selective catalytic reduction of NOx with methane. Journal of Catalysis, 237, 79–93. DOI: 10.1016/j.jcat.2005.09.036.
Teshima, N., Genfa, Z., & Dasgupta, P. K., (2004). Catalytic decomposition of hydrogen peroxide by a flow-through selfregulating platinum black heater. Analytica Chimica Acta, 510, 9–13. DOI: 10.1016/j.aca.2003.12.050.
Tsoncheva, T., Roggenbuck, J., Tiemann, M., Ivanova, L., Paneva, D., Mitov, I., & Minchev, C. (2008). Iron oxide nanoparticles supported on mesoporous MgO and CeO2: A comparative physicochemical and catalytic study. Microporous and Mesoporous Materials, 110, 339–346. DOI: 10.1016/j.micromeso.2007.06.021.
Turky, A. M. (2003). Electrical surface and catalytic properties of NiO as influenced by doping with CuO and Ag2O. Applied Catalysis A: General, 247, 83–93. DOI: 10.1016/s0926-860x(03)00089-9.
Turky, A. E. M., Radwan, N. R. E., & El-Shobaky, G. A. (2001). Surface and catalytic properties of CuO doped with MgO and Ag2O. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 181, 57–68. DOI: 10.1016/s0927-7757(00)00801-3.
Tzou, M. S., Teo, B. K., & Sachtler, W. M. H. (1988). Formation of Pt particles in Y-type zeolites: The influence of coexchanged metal cations. Journal of Catalysis, 113, 220–235. DOI: 10.1016/0021-9517(88)90250-3.
Wachs, I. E. (2005). Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catalysis Today, 100, 79–94. DOI: 10.1016/j.cattod.2004.12.019.
Wang, J. A., Novaro, O., Bokhimi, X., López, T., Gómez, R., Navarrete, J., Llanos, M. E., & López-Salinas, E. (1998). Characterizations of the thermal decomposition of brucite prepared by sol-gel technique for synthesis of nanocrystalline MgO. Materials Letters, 35, 317–323. DOI: 10.1016/s0167-577x(97)00273-5.
Weast, R. C. (1984). Handbook of chemistry and physics (65th ed., pp. 165). Boca Raton, FL, USA: CRC Press.
Weckhuysen, B. M., Heidler, R., & Schoonheydt, R. A. (2004). Electron spin resonance spectroscopy. Molecular Sieves — Science and Technology, 4, 2733. DOI: 10.1007/b94238.
Wee, J. H. (2006). Which type of fuel cell is more competitive for portable application: Direct methanol fuel cells or direct borohydride fuel cells? Journal of Power Sources, 161, 1–10. DOI: 10.1016/j.jpowsour.2006.07.032.
Wu, Y., Yan, A., He, Y., Wu, B., & Wu, T. (2010). Ni-Ag-O as catalyst for a novel one-step reaction to convert ethane to ethylene oxide. Catalysis Today, 158, 258–262. DOI: 10.1016/j.cattod.2010.03.041.
Zheng, J., Schmauke, T., Roduner, E., Dong, J. L., & Xu, Q. H. (2001). The influence of Fe on the dispersion, electronic state, sulfur-resistance and catalysis of platinum supported on KL zeolite. Journal of Molecular Catalysis A: Chemical, 171, 181–190. DOI: 10.1016/s1381-1169(01)00096-6.
Zielińska-Jurek, A., Kowalska, E., Sobczak, J. W., Lisowski, W., Ohtani, B., & Zaleska, A. (2011). Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Applied Catalysis B: Environmental, 101, 504–514. DOI: 10.1016/j.apcatb.2010.10.022.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Molla, S.A., Ali, L.I., Amin, N.H. et al. Effect of Ag-doping of nanosized FeMgO system on its structural, surface, spectral, and catalytic properties. Chem. Pap. 66, 722–732 (2012). https://doi.org/10.2478/s11696-012-0183-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-012-0183-x