Abstract
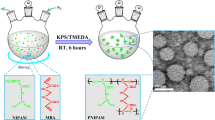
A number of poly(N-isopropylacrylamide) (polyNIPAM) microgels were prepared with dimethacrylate cross-linking agents of various lengths, ether and ester groups in the backbone, and pendant vinylidine functionality. These materials were characterized by examining their morphological patterns using optical and scanning electron microscopy. When ethylene glycol dimethacrylate (EGDMA) was used as a cross-linking agent, microspheres of approximately 1 μm in diameter were obtained. Diethylene glycol dimethacrylate (DEGDMA) cross-linking resulted in relatively large spherical structures (1–5 μm) as well as spherical nanostructures (200 nm). Using triethylene glycol dimethacrylate (TEGDMA) resulted in spheres with diameters between 1 μm and 3 μm. The hydrodynamic particle diameter decreased with the increasing chain length of the dimethacrylate cross-linking agents. The turbidity increased with the temperature of transition points occurring at approximately 31–32°C confirming the thermosensitivity of the obtained polymeric structures.
Similar content being viewed by others
References
Ankareddi, I., & Brazel, C. S. (2007). Synthesis and characterization of grafted thermosensitive hydrogels for heating activated controlled release. International Journal of Pharmaceutics, 336, 241–247. DOI: 10.1016/j.ijpharm.2006.11.065.
Arima, T., Hamada, T., & McCabe, J. F. (1995). The effects of cross-linking agents on some properties of HEMA-based resins. Journal of Dental Research, 74, 1597–1601. DOI: 10.1177/00220345950740091501.
Barszczewska-Rybarek, I.-M. (2009). Structure-property relationships in dimethacrylate networks based on Bis-GMA, UDMA and TEGDMA. Dental Materials, 25, 1082–1089. DOI: 10.1016/j.dental.2009.01.106.
Bobofchak, K. M., Tarasov, E., & Olsen, K. W. (2008). Effect of cross-linker length on the stability of hemoglobin. Biochimica et Biophysica Acta - Proteins & Proteomics, 1784, 1410–1414. DOI: 10.1016/j.bbapap.2008.01.014.
Brazel, C. S., & Peppas, N. A. (1996). Pulsatile and local delivery of methrombolytic and antithrombotic agents using poly(N-isopropylacrylamide-co-methacrylic acid) hydrogels. Journal of Controlled Release, 39, 57–64. DOI: 10.1016/0168-3659(95)00134-4.
Brazel, C. S., & Peppas, N. A. (1994). Temperature- and pH-sensitive hydrogels for controlled release of heparin and streptoki- nase. In A. G. Mikos, R. M. Murphy, H. Bernstein, & N. A. Peppas (Eds.), Biomaterials for drug and cell delivery (pp. 211–216). Pittsburgh, PA, USA: Materials Research Society.
Çaykara, T., Kiper, S., Demirel, G., Demirci, S., & Çakanyıldırım, Q. (2007). Temperature-responsive characteristics of poly(N-isopropylacrylamide) hydrogels with macroporous structure. Polymer International, 56, 275–282. DOI: 10.1002/pi.2162.
Ciullo, P. A. (1996). Rubber. In P. A. Ciullo (Ed.), Industrial minerals and their uses: A handbook & formulary (p. 220). New Jersey, NJ, USA: Noyes Publications.
Cornelius, V. J., Snowden, M. J., Silver, J., & Fern, G. R. (2004). A study of the binding of the biologically important hematin molecule to a novel imidazole containing poly(N-isopropylacrylamide) microgel. Reactive and Functional Polymers, 58, 165–173. DOI: 10.1016/j.reactfunct polym.2003.12.003.
Coughlan, D. C., & Corrigan, O. I. (2006). Drug-polymer interactions and their effect on thermoresponsive poly(N-isopropylacrylamide) drug delivery systems. International Journal of Pharmaceutics, 313, 163–174. DOI: 10.1016/j. ijpharm.2006.02.005.
Dale, J. A., & Millar, J. R. (1981). Cross-linker effectiveness in styrene copolymerization. Macromolecules, 14, 1515–1518. DOI: 10.1021/ma50006a072.
Das, M., Zhang, H., & Kumacheva, E. (2006). Microgels: Old materials with new applications. Annual Review of Material Research, 36, 117–142. DOI: 10.1146/annurev.matsci.36.011205.123513.
Duan, Q., Narumi, A., Miura, Y., Shen, X., Sato, S.-I., Satoh, T., & Kakuchi, T. (2006). Thermoresponsive property controlled by end-functionalization of poly(N-isopropylacrylamide) with phenyl, biphenyl, and triphenyl groups. Polymer Journal, 38, 306–310. DOI: 10.1295/polymj.38.306.
Ganapathy, S., Rajamohanan, P. R., Badiger, M. V., Mandhare, A. B., & Mashelkar, R. A. (2000). Proton magnetic resonance imaging in hydrogels: volume phase transition in poly(N-isopropylacrylamide). Polymer, 41, 4543–4547. DOI: 10.1016/S0032-3861(99)00615-1.
Guerrero-Ramírez, L. G., Nuńo-Donlucas, S. M., Cesteros, L. C., & Katimea, I. (2008). Smart copolymeric nanohydrogels: Synthesis, characterization and properties. Materials Chemistry and Physics, 112, 1088–1092. DOI: 10.1016/j.matchem phys.2008.07.023.
Hamerska-Dudra, A., Bryjak, J., & Trochimczuk, A. W. (2006). Novel method of enzymes stabilization on crosslinked thermosensitive carriers. Enzyme and Microbial Technology, 38, 921–925. DOI: 10.1016/j.enzmictec.2005.08.019.
Haselgrübler, T., Amerstorfer, A., Schindler, H., & Gruber, H. J. (1995). Synthesis and applications of a new poly(ethy1ene glycol) derivative for the crosslinking of amines with thiols. Bioconjugate Chemistry, 6, 242–248. DOI: 10.1021/bc00033a002.
Hirose, Y., Amiya, T., Hirokawa, Y., & Tanaka, T. (1987). Phase transition of submicron gel beads. Macromolecules, 20, 1342–1344. DOI: 10.1021/ma00172a029.
Hoare, T., & McLean, D. (2006). Kinetic prediction of functional group distributions in thermosensitive microgels. The Journal of Physical Chemistry B, 110, 20327–20336. DOI: 10.1021/jp0643451.
Hoare, T., & Pelton, R. (2007). Functionalized microgel swelling: Comparing theory and experiment. The Journal of Physical Chemistry B, 111, 11895–11906. DOI: 10.1021/jp072360f.
Iizawa, T., Ishido, T., Gotoh, T., & Sakohara, S. (2007). Synthesis of nonporous poly(N-alkylacrylamide) gel beads by nonaqueous sedimentation polymerization. Polymer Journal, 39, 18–20. DOI: 10.1295/polymj.PJ2006097.
Kim, J.-W., Utada, A. S., Fernández-Nieves, A., Hu, Z., & Weitz, D. A. (2007). Fabrication of monodisperse gel shells and functional microgels in microfluidic devices. Angewandte Chemie International Edition, 46, 1819–1822. DOI: 10.1002/anie.200604206.
Kiritoshi, Y., & Ishihara, K. (2003). Molecular recognition of alcohol by volume phase transition of cross-linked poly(2-methacryloyloxyethyl phosphorylcholine) gel. Science and Technology of Advanced Materials, 4, 93–98. DOI: 10.1016/S1468-6996(03)00010-X.
Kalagasidis Krušić, M. K., Dzunuzović, E., Trifunović, S., & Filipović, J. (2003). Semi-IPNs based on polyacrylamide and poly(itaconic acid). Polymer Bulletin, 51, 159–166. DOI: 10.1007/s00289-003-0203-7.
Li, L., & Lee, L. J. (2005). Photopolymerization of HEMA/DEGDMA hydrogels in solution. Polymer, 46, 11540–11547. DOI: 10.1016/j.polymer.2005.10.051.
Lindman, S., Lynch, I., Thulin, E., Nilsson, H., Dawson, K. A., & Linse, S. (2007). Sysyematic investigation of the thermodynamics of HSA adsorption to N-iso-propylacrylamide/N-tert-butylacrylamide copolymer nanoparticles. Effect of particle size and hydrophobicity. NanoLetters, 7, 914–920. DOI: 10.1021/nl062743+.
Ma, X., Xi, J., Huang, X., Zhao, X., & Tang, X. (2004). Novel hydrophobically modified temperature-sensitive microgels with tunable volume-phase transition temperature. Materials Letters, 58, 3400–3404. DOI: 10.1016/j.matlet.2004.04.019.
Maeda, T., Kanda, T., Yonekura, Y., Yamamoto, K., & Aoyagi, T. (2006). Hydroxylated poly(N-isopropylacrylamide) as functional thermoresponsive materials. Biomacromolecules, 7, 545–549. DOI: 10.1021/bm050829b.
Mathias, L. J., & Dickerson, C. W. (1991). Acrylate-containing oligo(ether-ester) cross-linking agents with controlled molecular weights via end-group termination. Macromolecules, 24, 2048–2053. DOI: 10.1021/ma00008a052.
Musial, W., Vincent, B., Szumny, A., & Voncina, B. (2010). Morphological characteristics of modified freeze-dried poly (N-isopropylacrylamide) microspheres studied by optical microscopy, SEM, and DLS. Chemical Papers, 64, 602–612. DOI: 10.2478/s11696-010-0041-7.
Nolan, C. M., Gelbaum, L. T., & Lyon, L. A. (2006). 1H NMR investigation of thermally triggered insulin release from poly(N-isopropylacrylamide) microgels. Biomacromolecules, 7, 2918–2922. DOI: 10.1021/bm060718s.
Oyerokun, F. T., & Schweizer, K. S. (2005). Thermodynamics, orientational order and elasticity of strained liquid crystalline melts and elastomers. The Journal of Physical Chemistry B, 109, 6595–6603. DOI: 10.1021/jp045646i.
Pelton, R. H., & Chibante, P. (1986). Preparation of aqueous lattices with N-isopropylacrylamide. Colloids and Surfaces, 20, 247–256. DOI: 10.1016/0166-6622(86)80274-8.
Ringsdorf, H., Venzmer, J., & Winnik, F. M. (1991). Fluorescence studies of hydrophobically modified poly(N-isopropylacrylamides). Macromolecules, 24, 1678–1686. DOI: 10.1021/ma00007a034.
Saunders, B. R., Laajam, N., Daly, E., Teow, S., Hu, X., & Stepto, R. (2009). Microgels: From responsive polymer colloids to biomaterials. Advances in Colloid and Interface Science, 147–148, 251–262. DOI: 10.1016/j.cis.2008.08.008.
Schild, H. G. (1992). Poly(N-isopropylacrylamide): experiment, theory and application. Progress in Polymer Science, 17, 163–249. DOI: 10.1016/0079-6700(92)90023-R.
Serres, A., Baudyš, M., & Kim, S. W. (1996). Temperature and pH-sensitive polymers for human calcitonin delivery. Pharmaceutical Research, 13, 196–201. DOI: 10.1023/A:1016026711364.
Shefer, A., Grodzinsky, A. J., Prime, K. L., & Busnel, J.-P. (1993). Novel model networks of poly(acry1ic acid): Synthesis and characterization. Macromolecules, 26, 5009–5014. DOI: 10.1021/ma00071a004.
Shen, Z., Wei, W., Zhao, Y., Ma, G., Dobashi, T., Maki, Y., Su, Z., & Wan, J. (2008). Thermosensitive polymer-conjugated albumin nanospheres as thermal targeting anti-cancer drug carrier. European Journal of Pharmaceutical Sciences, 35, 271–282. DOI: 10.1016/j.ejps.2008.07.006.
Skrabania, K., Kristen, J., Laschewsky, A. Akdemir, Ö., Hoth, A., & Lutz, J.-F. (2007). Design, synthesis, and aqueous aggregation behavior of nonionic single and multiple thermoresponsive polymers. Langmuir, 23, 84–93. DOI: 10.1021/la061509w.
Snowden, M. J., & Vincent, B. (1992). The temperature-controlled flocculation of crosslinked latex particles. Journal of the Chemical Society, Chemical Communications, 1992, 1103–1105. DOI: 10.1039/C39920001103.
Soppimath, K. S., Tan, D. C.-W., & Yang, Y. Y. (2005). pH-triggered thermally responsive polymer core-shell nanoparticles for drug delivery. Advanced Materials, 17, 318–323. DOI: 10.1002/adma.200401057.
Suzuki, A. (1993). Phase transition in gels of sub-millimeter size induced by interaction with stimuli. In Advances in Polymer Science (Vol. 110), Responsive Gels: Volume Transitions II (pp. 199–240). Berlin/Heidelberg, Germany: Springer. DOI: 10.1007/BFb0021134.
Suzuki, A., & Tanaka, T. (1990). Phase transition in polymer gels induced by visible light. Nature, 346, 345–347. DOI: 10.1038/346345a0.
Tanaka, F., Koga, T., & Winnik, F. (2008). Temperature-responsive polymers in mixed solvents: Competitive hydrogen bonds cause cononsolvency. Physical Review Letters, 101, 028302–1–028302–4. DOI: 10.1103/PhysRevLett.101.028302.
Wei, H., Zhang, X.-Z. Chen, W.-Q., Cheng, S.-X., & Zhuo, R.-X. (2007). Self-assembled thermosensitive micelles based on poly(l-lactide-star block-N-isopropylacrylamide) for drug delivery. Journal of Biomedical Materials Research Part A, 83A, 980–989. DOI: 10.1002/jbm.a.31295.
Xu, Y., Du, Y., Huang, R., & Gao, L. (2004). Preparation and modification of N-(2-hydroxyl)propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials, 24, 5015–5022. DOI: 10.1016/S0142-9612(03)00408-3.
Zhou, Y.-M., Ishikawa, A., Okahashi, R., Uchida, K., Nemoto, Y., Nakayama, M., & Nakayama, Y. (2007). Deposition transfection technology using a DNA complex with a thermoresponsive cationic star polymer. Journal of Controlled Release, 123, 239–246. DOI: 10.1016/j.jconrel.2007.08.026.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musial, W., Kokol, V., Fecko, T. et al. Morphological patterns of poly(N-isopropylacrylamide) derivatives synthesized with EGDMA, DEGDMA, and TEGDMA crosslinkers for application as thermosensitive drug carriers. Chem. Pap. 64, 791–798 (2010). https://doi.org/10.2478/s11696-010-0065-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-010-0065-z