Abstract
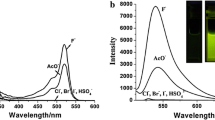
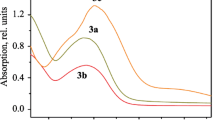
Effect of the addition of six different anions on the absorption and fluorescence spectra of acridine yellow G (AYG) was examined. Only the F− anion could induce a visible color change observable with naked eye and a strong fluorescence quenching with K SV of 8.3 × 104 mol−1 L in CH3CN solutions. Calculated results of the interaction between the F− anion and acridine yellow G using the B3LYP/6-31G(d) program showed that the intermolecular charge transfer through the formation of an H-bond between AYG and F− is an essential sensor mechanism.
Similar content being viewed by others
References
Ayling, A. J., Pérez-Payán, M. N., & Davis, A. P. (2001). New “cholapod” anionophores; high-affinity halide receptors derived from cholic acid. Journal of the American Chemical Society, 123, 12716–12717. DOI: 10.1021/ja016796z.
Barboiu, M., Vaughan, G., & van der Lee, A. (2003). Self-organized heteroditopic macrocyclic superstructures. Organic Letters, 5, 3073–3076. DOI: 10.1021/ol035096r.
Beer, P. D., & Gale, P. A. (2001). Anion recognition and sensing: The state of the art and future perspectives. Angewandte Chemie International Edition, 40, 486–516. DOI: 10.1002/1521-3773(20010202).
Bondy, C. R., & Loeb, S. J. (2003). Amide based receptors for anions. Coordination Chemistry Reviews, 240, 77–99. DOI: 10.1016/S0010-8545(02)00304-1.
Camiolo, S., Gale, P. A., Hursthouse, M. B., & Light, M. E. (2003). Nitrophenyl derivatives of pyrrole 2,5-diamides: structural behaviour, anion binding and colour change signalled deprotonation. Organic & Biomolecular Chemistry, 1, 741–744. DOI: 10.1039/b210848h.
Camiolo, S., Gale, P. A., Hursthouse, M. B., Light, M. E., & Shi, A. J. (2002). Solution and solid-state studies of 3,4-dichloro- 2,5-diamidopyrroles: formation of an unusual anionic narcissistic dimer. Chemical Communications, 2002, 758–759. DOI: 10.1039/b200980c.
Chmielewski, M., & Jurczak, J. (2004). Size complementarity in anion recognition by neutral macrocyclic tetraamides. Tetrahedron Letters, 45, 6007–6010. DOI: 10.1016/j.tetlet.2004.06.037.
Davis, A. P. (1999). Steroidal guanidinium receptors for the enantioselective recognition of N-acyl α-amino acids. Chemical Communications, 1999, 9–10. DOI: 10.1039/a808245f.
Davis, A. P., Perry, J. J., & Williams, R. P. (1997). Anion recognition by tripodal receptors derived from cholic acid. Journal of the American Chemical Society, 119, 1793–1794. DOI: 10.1021/ja9629930.
de Silva, A. P., Fox, D. B., Huxley, A. J. M., & Moody, T. S. (2000). Combining luminescence, coordination and electron transfer for signalling purposes. Coordination Chemistry Reviews, 205, 41–57. DOI: 10.1016/S0010-8545(00)00238-1.
Esteban-Gómez, D., Fabbrizzi, L., & Licchelli, M. (2005a). Why, on interaction of urea-based receptors with fluoride, beautiful colors develop. The Journal of Organic Chemistry, 70, 5717–5720. DOI: 10.1021/jo050528s.
Esteban-Gómez, D., Fabbrizzi, L., Licchelli, M., & Sacchi, D. (2005b). A two-channel chemosensor for the optical detection of carboxylic acids, including cholic acid. Journal of Materials Chemistry, 15, 2670–2675. DOI: 10.1039/b502869h.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Montgomery, J. A., Jr., Vreven, T., Kudin, K. N., Burant, J. C., Millam, J. M., Iyengar, S. S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G. A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J. E., Hratchian, H. P., Cross, J. B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., W., Ochterski, J., Ayala, P. Y., Morokuma, K., Voth, G. A., Salvador, P., Dannenberg, J. J., Zakrzewski, V. G., Dapprich, S., Daniels, A. D., Strain, M. C., Farkas, O., Malick, D. K., Rabuck, A. D., Raghavachari, K., Foresman, J. B., Ortiz, J. V., Cui, Q., Baboul, A. G., Clifford, S., Cioslowski, J., Stefanov, B. B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R. L., Fox, D. J., Keith, T., Al-Laham, M. A., Peng, C. Y., Nanayakkara, A., Challacombe, M., Gill, P. M. W., Johnson, B., Chen, W., Wong, M. W., Gonzalez, C., & Pople, J. A. (2003). Gaussian 03, Revision B.05 [computer software]. Pittsburgh, PA, USA: Gaussian, Inc.
Gale, P. A. (2006). Structural and molecular recognition studies with acyclic anion receptors. Accounts of Chemical Research, 39, 465–475. DOI: 10.1021/ar040237q.
Gale, P. A. (2003). Anion and ion-pair receptor chemistry: Highlights from 2000 and 2001. Coordination Chemistry Reviews, 240, 191–221. DOI: 10.1016/S0010-8545(02)00258-8.
Gale, P. A., & Quesada, R. (2006). Anion coordination and anion-templated assembly: Highlights from 2002 to 2004. Coordination Chemistry Reviews, 250, 3219–3244. DOI: 10.1016/j.ccr.2006.05.020.
Gunnlaugsson, T. (2001). A novel Eu(III)-based luminescent chemosensor: determining pH in a highly acidic environment. Tetrahedron Letters, 42, 8901–8905. DOI: 10.1016/S0040-4039(01)01935-9.
Gunnlaugsson, T., Davis, A. P., Hussey, G. M., Tierney, J., & Glynn, M. (2004). Design, synthesis and photophysical studies of simple fluorescent anion PET sensors using charge neutral thiourea receptors. Organic & Biomolecular Chemistry, 2, 1856–1863. DOI: 10.1039/b404706k.
Gunnlaugsson, T., Glynn, M., Tocci, G. M., Kruger, P. E., & Pfeffer, F. M. (2006). Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coordination Chemistry Reviews, 250, 3094–3117. DOI: 10.1016/j.ccr.2006.08.017.
Gunnlaugsson, T., Kruger, P. E., Jensen, P., Pfeffer, F. M., & Hussey, G. M. (2003). Simple naphthalimide based anion sensors: deprotonation induced colour changes and CO2 fixation. Tetrahedron Letters, 44, 8909–8913. DOI: 10.1016/j.tetlet.2003.09.148.
Gunnlaugsson, T., Kruger, P. E., Jensen, P., Tierney, J., Ali, H. D. P., & Hussey, G. M. (2005). Colorimetric “naked eye” sensing of anions in aqueous solution. Journal of Organic Chemistry, 70, 10875–10878. DOI: 10.1021/jo0520487.
Gunnlaugsson, T., Mac Dónaill, D. A., & Parker, D. (2001). Lanthanide macrocyclic quinolyl conjugates as luminescent molecular-level devices. Journal of the American Chemical Society, 123, 12866–12876. DOI: 10.1021/ja004316i.
Gunnlaugsson, T., Nieuwenhuyzen, M., Richard, L., & Thoss, V. (2002). Novel sodium-selective fluorescent PET and optically based chemosensors: towards Na+ determination in serum. Journal of Chemical Society, Perkin Transactions, 2, 141–150. DOI: 10.1039/b106474f.
Hettche, F., & Hoffmann, R. W. (2003). A tri-armed sulfonamide host for selective binding of chloride. New Journal of Chemistry, 27, 172–177. DOI: 10.1039/b206125m.
Kang, S. O., Hossain, M. A., & Bowman-James, K. (2006). Influence of dimensionality and charge on anion binding in amide-based macrocyclic receptors. Coordination Chemistry Reviews, 250, 3038–3052. DOI: 10.1016/j.ccr.2006.06.006.
Katayev, E. A., Sessler, J. L., Khrustalev, V. N., & Ustynyuk, Y. A. (2007). Synthetic model of the phosphate binding protein: solid-state structure and solution-phase anion binding properties of a large oligopyrrolic macrocycle. The Journal of Organic Chemistry, 72, 7244–7252. DOI: 10.1021/jo071106g.
Kim, S. H., Kim, J. S., Park, S. M., & Chang, S. K. (2006). Hg2+-selective OFF-ON and Cu2+-selective ON-OFF type fluoroionophore based upon cyclam. Organic Letters, 8, 371–374. DOI: 10.1021/ol052282j.
Kondo, S., Hiraoka, Y., Kurumatani, N., & Yano, Y. (2005). Selective recognition of dihydrogen phosphate by receptors bearing pyridyl moieties as hydrogen bond acceptors. Chemical Communications, 2005, 1720–1722. DOI: 10.1039/b417304j.
Lakowicz, J. R. (1999). Principles of fluorescence spectroscopy (2nd ed.). New York, NY, USA: Plenum Press.
Lee, D. H., Lee, H. Y., & Hong, J.-I. (2002). Anion sensor based on the indoaniline-thiourea system. Tetrahedron Letters, 43, 7273–7276. DOI: 10.1016/S0040-4039(02)01455-7.
Lee, C., Miyaji, H., Yoon, D., & Sessler, J. L. (2008). Strapped and other topographically nonplanar calixpyrrole analogues. Improved anion receptors. Chemical Communications, 2008, 24–34. DOI: 10.1039/b713183f.
Liao, J.-H., Chen, C.-T., & Fang, J.-M. (2002). A novel phosphate chemosensor utilizing anion induced fluorescence change. Organic Letters, 4, 561–564. DOI: 10.1021/ol0171564.
Liu, B., & Tian, H. (2005). A ratiometric fluorescent chemosensor for fluoride ions based on a proton transfer signaling mechanism. Journal of Materials Chemistry, 15, 2681–2686. DOI: 10.1039/b501234a.
Luxami, V., Sharma, N., & Kumar, S. (2008). Quaternary ammonium salt-based chromogenic and fluorescent chemosensors for fluoride ions. Tetrahedron Letters, 49, 4265–4268. DOI: 10.1016/j.tetlet.2008.04.147.
Mikláš, R., Kasák, P., Devínsky, F., & Putala, M. (2009). Fluoride anion sensing using colorimetric reagents containing binaphthyl moiety and urea binding site. Chemical Papers, 63, 709–715. DOI: 10.2478/s11696-009-0079-6.
Miyaji, H., & Sessler, J. L. (2001). Off-the-shelf colorimetric anion sensors. Angewandte Chemie International Edition, 40, 154–157. DOI: 10.1002/1521-3773.
Peng, X., Wu, Y., Fan, J., Tian, M., & Han, K. (2005). Colorimetric and ratiometric fluorescence sensing of fluoride: Tuning selectivity in proton transfer. Journal of Organic Chemistry, 70, 10524–10531. DOI: 10.1021/jo051766q.
Rurack, K., & Resch-Genger, U. (2002). Rigidization, preorientation and electronic decoupling—the magic triangle for the design of highly efficient fluorescent sensors and switches. Chemical Society Reviews, 31, 116–127. DOI: 10.1039/b100604p.
Sessler, J. L., Cho, W.-S., Gross, D. E., Shriver, J. A., Lynch, V. M., & Marquez, M. (2005). Anion binding studies of fluorinated expanded calixpyrroles. The Journal of Organic Chemistry, 70, 5982–5986. DOI: 10.1021/jo050662c.
Sessler, J. L., Gross, D. E., Cho, W.-S., Lynch, V. M., Schmidtchen, F. P., & Bates, G.W. (2006). Calix[4]pyrrole as a chloride anion receptor: solvent and countercation effects. Journal of the American Chemical Society, 128, 12281–12288. DOI: 10.1021/ja064012h.
Suksai, C., & Tuntulani, T. (2003). Chromogenic anion sensors. Chemical Society Reviews, 32, 192–202. DOI: 10.1039/b209598j.
Tseng, Y., Tu, G., Lin, C., Chang, C., Lin, C., & Yen, Y. (2007). Synthesis of colorimetric sensors for isomeric dicarboxylate anions: selective discrimination between maleate and fumarate. Organic & Biomolecular Chemistry, 5, 3592–3598. DOI: 10.1039/b710695e.
Yen, Y.-P., & Ho, K.-W. (2006). Synthesis of colorimetric receptors for dicarboxylate anions: a unique color change for malonate. Tetrahedron Letters, 47, 1193–1196. DOI: 10.1016/j.tetlet.2005.12.009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, P., Guo, F., Wang, W. et al. A naked-eye, selective and sensitive chemosensor for fluoride ion. Chem. Pap. 64, 723–728 (2010). https://doi.org/10.2478/s11696-010-0060-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-010-0060-4