Abstract
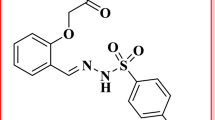
A deuterium-palladium electrode was employed as a new indicator electrode for the titration of weak acids in acetonitrile and benzonitrile. The investigated electrode showed a linear dynamic response for p-toluenesulfonic acid in the concentration range from 0.001 M to 0.1 M with a Nernstian slope of 48 mV in acetonitrile. Sodium methylate, potassium hydroxide and tetrabutylammonium hydroxide proved to be very suitable titrating agents for these titrations. The response time was less than 10-11 s and the lifetime of the electrode was limitless. Advantages of the electrode are: long-term stability, fast response, reproducibility, easy preparation and low cost.
Similar content being viewed by others
References
Aktaş, A. H., Yaşar, G., & Alsancak, G. Ö. (2001). Conductimetric and potentiometric titration of some hydroxylated cinnamic acids with tetrabutylammonium hydroxide in nonaqueous media. Turkish Journal of Chemistry, 25, 501–508.
Al-Daher, I. M., & Kratochvil, B. (1980). Potentiometric titrations of metal ions in acetonitrile with polyamine ligands. Talanta, 27, 983–988. DOI: 10.1016/0039-9140(80)80131-7.
Andrés, M. J., & Romero, C. (1988). Differentiation of the acidic groups of fulvic acids from lignite by potentiometric titration in acetone, acetonitrile and isopropanol. Fuel, 67, 1305–1307. DOI: 10.1016/0016-2361(88)90055-5.
Antonijević, M. M., Vukanović, B., & Mihajlović, R. (1992). Natural monocrystalline pyrite as electrode material for potentiometric titrations in water. Talanta, 39, 809–814. DOI: 10.1016/0039-9140(92)80100-R.
Barbosa, J., Hernandez-Cassou, S., Sanz-Nebot, V., & Toro, I. (1997). Variation of acidity constants of peptides in acetonitrile-water mixtures with solvent composition: effect of preferential solvation. The Journal of Peptide Research, 50, 14–24. DOI: 10.1111/j.1399-3011.1997.tb00615.x.
Barbosa, J., Roses, M., & Sanz-Nebot, V. (1988). Acid-base indicators in acetonitrile: Their pK values and chromatic parameters. Talanta, 35, 1013–1018. DOI: 10.1016/0039-9140(88)80240-6.
Barbosa, J., & Sanz-Nebot, V. (1989). Acid-base equilibria and assay of benzodiazepines in acetonitrile medium. Talanta, 36, 837–842. DOI: 10.1016/0039-9140(89)80164-X.
Barbosa, J., Sanz-Nebot, V., & Torrero, E. (1991). Equilibrium constants and assay of bases in acetonitrile. Talanta, 38, 425–432. DOI:10.1016/0039-9140(91)80081-A.
Barbosa, J., Sanz-Nebot, V., & Torrero, M. E. (1990). Acid-base equilibria of β-blockers in acetonitrile. Journal of Pharmaceutical and Biomedical Analysis, 8, 675–679. DOI: 10.1016/0731-7085(90)80101-T.
Bartnicka, H., Bojanowska, I., & Kalinowski, M. K. (1991). Solvent effect on the dissociation constants of aliphatic carboxylic acids. Australian Journal of Chemistry, 44, 1077–1084. DOI: 10.1071/CH9911077.
Bates, R. G. (1973). Determination of pH; theory and practice. New York, NY, USA: Wiley.
Chasemi, J., Ahmadi, S., Kubista, M., & Forootan, A. (2003). Determination of acidity constants of 4-(2-pyridylazo)resorcinol in binary acetonitrile + water mixtures. Journal of Chemical & Engineering Data, 48, 1178–1182. DOI: 10.1021/je030116l.
Czerwiński, A., Marassi, R., & Zamponi, S. (1991). The absorption of hydrogen and deuterium in thin palladium electrodes: Part I. Acidic solutions. Journal of Electroanalytical Chemistry, 316, 211–221. DOI: 10.1016/0022-0728(91)87047-8.
Ertekin, K., Alp, S., & Yalcın, I. (2004). Determination of pK a values of azlactone dyes in non-aqueous media. Dyes and Pigments, 65, 33–38. DOI: 10.1016/j.dyepig.2004.06.011.
Fleischmann, M., & Pons, S. (1989). Electrochemically induced nuclear fusion of deuterium. Journal of Electroanalytical Chemistry, 261, 301–308. DOI: 10.1016/0022-0728(89)80006-3.
Galster, H. (1990). pH-Messung: Grundlagen, Methoden, Anwendungen, Geräte. Weinheim, Germany: Wiley-VCH.
Greenhow, E. J., & Al-Mudarris, B. F. (1975). Metal and metalloid indicator electrodes for the non-aqueous potentiometric titration of weak acids: Comparative evaluation of group III, IV and V main-group elements. Talanta, 22, 417–424. DOI:10.1016/0039-9140(75)80089-0.
Gündüz, T., Gündüz, N., Kılıc, E., Köseoğlu, F., & Öztas, S. G. (1988). Titrations in non-aqueous media. Part X. Potentiometric and conductiometric titrations of amino acids with tetrabutylammonium hydroxide in pyridine and acetonitrile solvents. Analyst, 113, 715–719. DOI: 10.1039/AN9881300715.
Gündüz, T., Kiliç, E., Özkan, G., Awaad, M. F., & Tastekin, M. (1990). Conductimetric and potentiometric investigation of effect of acidity on formation of homoconjugates in acetonitrile solvent. Canadian Journal of Chemistry, 68, 674–678. DOI: 10.1139/v90-103.
Harlow, G. A., Noble, C. M., & Wyld, G. E. A. (1956). Potentiometric titration of very weak acids. Titration in ethylenediamine solution using platinum electrodes. Analytical Chemistry, 28, 784–786. DOI: 10.1021/ac60113a002.
Herrador, M. Á., & González, A. G. (2002). Potentiometric titrations in acetonitrile-water mixtures: evaluation of aqueous ionisation constant of ketoprofen. Talanta, 56, 769–775. DOI: 10.1016/S0039-9140(01)00607-5.
Hoare, J. P. (1959). Surface to volume considerations in the palladium-hydrogen-acid system. Journal of the Electrochemical Society, 106, 640–643. DOI: 10.1149/1.2427462.
Hoare, J. P., & Schuldiner, S. (1957). Effects of hydrogen content on the resistance and the potential in the palladium-hydrogen-acid system. The Journal of Physical Chemistry, 61, 399–402. DOI: 10.1021/j150550a004.
Hojo, M., & Chen, Z., (1999). Appearance of maxima on conductometric titration curves of sulfonic acids and the evidence of strong homoconjugation reactions in benzonitrile. Analytical Sciences, 15, 303–306. DOI: 10.2116/analsci.15.303.
Izutsu, K., Nakamura, T., Arai, T., & Ohmaki, M. (1995). Some recent studies on the use of electrochemical sensors in nonaqueous solution chemistry. Electroanalysis, 7, 884–888. DOI: 10.1002/elan.1140070916.
Izutsu, K., & Ohmaki, M. (1996). Acid-base equilibria in γ-butyrolactone studied by use of pH-ISFETs. Talanta, 43, 643–648. DOI: 10.1016/0039-9140(95)01799-2.
Izutsu, K., & Yamamoto, H. (1996). Response of an iridium oxide pH-sensor in nonaqueous solutions. Comparison with other pH-sensors. Analytical Sciences, 12, 905–909. DOI: 10.2116/analsci.12.905.
Karlberg, B., & Johansson, G. (1969). Alkaline errors of glass electrodes in non-aqueous solvents. Talanta, 16, 1545–1551. DOI: 10.1016/0039-9140(69)80215-8.
Katz, M., & Glenn, R. A. (1952). Sodium aminoethoxide titration of weak acids in ethylenediamine. Analytical Chemistry, 24, 1157–1163. DOI: 10.1021/ac60067a024.
Kolthoff, I. M., Bruckenstein, S., & Chantooni, M. K., Jr. (1961). Acid-base equilibria in acetonitrile. Spectrophotometric and conductometric determination of the dissociation of various acids. Journal of the American Chemical Society, 83, 3927–3935. DOI: 10.1021/ja01480a001.
Kolthoff, I. M., & Chantooni, M. K. (1966). Conductometric, potentiometric, and spectrophotometric determination of dissociation constants of substituted benzoic acids in acetonitrile. The Journal of Physical Chemistry, 70, 856–866. DOI: 10.1021/j100875a039.
Kolthoff, I. M., & Chantooni, M. K. (1965). Calibration of the glass electrode in acetonitrile. Shape of potentiometric titration curves. Dissociation constant of picric acid. Journal of the American Chemical Society, 87, 4428–4436. DOI: 10.1021/ja00948a004.
Kolthoff, I. M., & Chantooni, M. K., Jr. (1975). Titration in dipolar aprotic solvents of diprotic acids as monoprotic acids. Analytical Chemistry, 47, 1921–1926. DOI: 10.1021/ac60362a001.
Kolthoff, I. M., & Chantooni, M. K., Jr. (1969). Homoconjugation constant of picric acid in acetonitrile. The Journal of Physical Chemistry, 73, 4029–4030. DOI: 10.1021/j100845a084.
Kolthoff, I. M., Chantooni, M. K., Jr., & Bhowmik, S. (1966). Acid-base properties of mono- and dinitrophenols in acetonitrile. Journal of the American Chemical Society, 88, 5430–5439. DOI: 10.1021/ja00975a011.
Kreshkov, A. P., Bykova, L. N., & Kazaryan, N. A. (1967). Kislotno-osnovnoe titrovanie v nevodnykh rastvorakh. Moscow, USSR: Khimia.
Kurtoğlu, M., Birbiçer, N., Kimyonşen, Ü., & Serin, S. (1999). Determination of pK a values of some azo dyes in acetonitrile with perchloric acid. Dyes and Pigments, 41, 143–147. DOI: 10.1016/S0143-7208(98)00077-1.
Kuruoğlu, D., Canel, E., Memon, S., Yilmaz, M., & Kiliç, E. (2003). Hydrogen ion-selective poly(vinyl chloride) membrane electrode based on a calix[4]arene. Analytical Sciences, 19, 217–221. DOI: 10.2116/analsci.19.217.
Lewis, F. A., & Ubbelohde, A. R. (1954). Mechanisms of removal of hydrogen from palladium-hydrogen by oxidation. Journal of the Chemical Society, 1954, 1710–1716. DOI: 10.1039/JR9540001710.
Lintner, C. J., Schleif, R. H., & Higuchi, T. (1950). Electrometric titration of alcohols using lithium aluminium hydride. Analytical Chemistry, 22, 534–538. DOI: 10.1021/ac60040a007.
Mihajlović, L., Nikolić-Mandić, S., Vukanović, B., & Mihajlović, R., (2009). Use of the sulfide mineral pyrite as electrochemical sensor in non-aqueous solutions: Potentiometric titration of weak acids in acetonitrile, propionitrile and benzonitrile. Analytical Sciences, 25, 437–441. DOI: 10.2116/analsci.25.437.
Mihajlović, L. V., Mihajlović, R. P., Antonijević, M. M., & Vukanović, B. V. (2004). Natural monocrystalline pyrite as a sensor in non-aqueous solution: Part I: Potentiometric titration of weak acids in N,N-dimethylformamide, methylpyrrolidone and pyridine. Talanta, 64, 879–886. DOI: 10.1016/j.talanta.2004.03.061.
Mihajlović, R. P., Jakšić, L. N., & Vajgand, V. V. (1992). Coulometric titrations of bases in propylene carbonate using hydrogen—palladium and deuterium—palladium generator electrodes. Talanta, 39, 1587–1590. DOI: 10.1016/0039-9140(92)80188-J.
Mihajlović, R. P., & Stanić Z. D. (2005). Natural monocrystalline chalcopyrite and galena as electrochemical sensors in non-aqueous solvents. Part I: potentiometric titrations of weak acids in γ-butyrolactone and propylene carbonate. Journal of Solid State Electrochemistry, 9, 558–565. DOI: 10.1007/s10008-004-0591-0.
Mihajlović, R. P., Vajgand, V. J., & Džudović, R. M. (1991). The application of deuterium-palladium electrodes in the coulometric-potentiometric determination of bases in ketone media. Talanta, 38, 673–675. DOI: 10.1016/0039-9140(91)80155-S.
Oyama, N., Hirokawa, T., Yamaguchi, S., Ushizawa, N., & Shimomura, T. (1987). Hydrogen ion selective microelectrode prepared by modifying an electrode with polymers. Analytical Chemistry, 59, 258–262. DOI: 10.1021/ac00129a009.
Pissinis, D., Sereno, L. E., & Marioli, J. M. (2005). Multiwavelength spectrophotometric determination of propofol acidity constant in different acetonitrile-water mixtures. Journal of the Brazilian Chemical Society, 16, 1054–1060. DOI: 10.1590/S0103-50532005000600024.
Sanz-Nebot, V., Valls, I., Barbero, D., & Barbosa, J. (1997). Acid-base behavior of quinolones in aqueous acetonitrile mixtures. Acta Chemica Scandinavica, 51, 896–903. DOI: 10.3891/acta.chem.scand.51-0896.
Shirvington, P. J. (1967). The hydrolysis of some acidic metal cations in acetonitrile containing traces of water. Australian Journal of Chemistry, 20, 447–453. DOI: 10.1071/CH9670447.
Vajgand, V. J., Mihajlović, R. P., Džudović, R. M., & Jakšić, L. N. (1987). Coulometric titration of salts of strong mineral acids in acetic anhydride by application of a hydrogen/palladium electrode. Analytica Chimica Acta, 202, 231–236. DOI: 10.1016/S0003-2670(00)85919-X.
Verma, B. C., & Sood, R. K. (1979). Determination of mercaptopyrimidines with copper(II) in acetonitrile. Talanta, 26, 906–907. DOI: 10.1016/0039-9140(79)80278-7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pantić, I.Ɖ., Mihajlović, R.P. & Mihajlović, L.V. A deuterium-palladium electrode as a new sensor in non-aqueous solutions: potentiometric titration of weak acids in acetonitrile and benzonitrile. Chem. Pap. 64, 541–549 (2010). https://doi.org/10.2478/s11696-010-0044-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-010-0044-4