Abstract
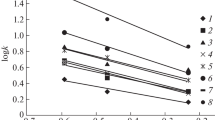
Preferential sorption, equilibrium swelling degree, and volume of the swollen membrane in systems low-density polyethylene + hexane + aromatic compound (benzene, methylbenzene, or ethylbenzene) were studied using gravimetric and dilatometric methods at the temperature of 25 °C. Aromatic compounds are preferentially sorbed in low-density polyethylene compared to hexane. Experimental volume changes of the membrane were found to be noticeably different from those calculated on the basis of mass sorption data under the assumption of additivity. This difference points to the existence of the interactions between the polymer and the surrounding liquid mixture. The volume swelling degree curves are close to each other in the whole concentration range of the binary liquid mixture (hexane + aromatics) suggesting that the attractive forces between the polymer chains are the deciding factor limiting the volume expansion of the membrane in all three systems to the same extent.
Similar content being viewed by others
References
Caceres Alonso, M., Arsuaga Ferreras, J. M., & Nuñez Delgado, J. (1985). Excess volumes of binary mixtures of ethylbenzene + n-alkanes. Fluid Phase Equilibria, 20, 81–85. DOI:10.1016/0378-3812(85)90023-8.
Friess, K., Jansen, J. C., Vopička, O., Randová, A., Hynek, V., Šípek, M., Bartovská, L., Izák, P., Dingemans, M., Dewulf, J., Van Langenhove, H., & Drioli, E. (2009). Comparative study of sorption and permeation techniques for the determination of heptane and toluene transport in polyethylene membranes. Journal of Membrane Science, 338, 161–174. DOI: 10.1016/j.memsci.2009.04.030.
Friess, K., Šípek, M., Hynek, V., Sysel, P., Bohatá, K., & Izák, P. (2004). Comparison of permeability coefficients of organic vapors through non-porous polymer membranes by two different experimental techniques. Journal of Membrane Science, 240, 179–185. DOI: 10.1016/j.memsci.2004.05.006.
Handa, Y. P., & Benson, G. C. (1979). Volume changes on mixing two liquids: A review of the experimental techniques and the literature data. Fluid Phase Equilibria, 3, 186–249. DOI: 10.1016/0378-3812(79)85010-4.
Heintz, A., & Stephan, W. (1994). A generalized solution-diffusion model of the pervaporation process through composite membranes Part I. Prediction of mixture solubilities in the dense active layer using the UNIQUAC model. Journal of Membrane Science, 89, 143–151. DOI: 10.1016/0376-7388(93)E0222-6.
Izák, P., Bartovská, L., Friess, K., Šípek, M., & Uchytil, P. (2003a). Description of binary liquid mixtures transport through non-porous membrane by modified Maxwell-Stefan equations. Journal of Membrane Science, 214, 293–309. DOI:10.1016/S0376-7388(02)00580-X.
Izák, P., Bartovská, L., Friess, K., Šípek, M., & Uchytil, P. (2003b). Comparison of various models for transport of binary mixtures through dense polymer membrane. Polymer, 44, 2679–2687. DOI: 10.1016/S0032-3861(03)00137-X.
Mulder, M. H. V., Franken, A. C. M., & Smolders, C. A. (1985). On the mechanism of separation of ethanol/water mixtures by pervaporation II. Experimental concentration profiles. Journal of Membrane Science, 23, 41–58. DOI: 10.1016/S0376-7388(00)83133-6.
Mulder, M. H. V., & Smolders, C. A. (1986). Pervaporation, solubility aspects of the solution-diffusion model. Separation & Purification Reviews, 15, 1–19. DOI: 10.1080/03602548608068423.
Mulder, M. H. V., & Smolders, C. A. (1984). On the mechanism of separation of ethanol/water mixtures by pervaporation I. Calculations of concentration profiles. Journal of Membrane Science, 17, 289–307. DOI: 10.1016/S0376-7388(00)83220-2.
National Institute of Standards and Technology, NIST (2008a). M. Frenkel (Ed.), TRC thermodynamic tables — Nonhydrocarbons (NSRDSNIST-74). Boulder, CO, USA: NIST.
National Institute of Standards and Technology, NIST (2008b). M. Frenkel (Ed.), TRC thermodynamic tables — Hydrocarbons (NSRDSNIST-75). Boulder, CO, USA: NIST.
Randová, A., Bartovská, L., Hovorka, Š., Friess, K., & Izák, P. (2009a). The membranes (Nafion and LDPE) in binary liquid mixtures benzene + methanol — sorption and swelling. European Polymer Journal, 45, 2895–2901. DOI: 10.1016/j.eurpolymj.2009.06.023.
Randová, A., Bartovská, L., Hovorka, Š., Poloncarzová, M., Kolská, Z., & Izák, P. (2009b). Application of the group contribution approach to Nafion swelling. Journal of Applied Polymer Science, 111, 1745–1750. DOI: 10.1002/app.29157.
Randová, A., Hovorka, Š., Izák, P., & Bartovská, L. (2008). Swelling of Nafion in methanol-water-inorganic salt ternary mixtures. Journal of Electroanalytical Chemistry, 616, 117–121. DOI: 10.1016/j.jelechem.2007.12.018.
Wijmans, J. G., & Baker, R. W. (1995). The solution-diffusion model: a review. Journal of Membrane Science, 107, 1–21. DOI: 10.1016/0376-7388(95)00102-I.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Randová, A., Bartovská, L., Hovorka, Š. et al. Low-density polyethylene in mixtures of hexane and benzene derivates. Chem. Pap. 64, 652–656 (2010). https://doi.org/10.2478/s11696-010-0038-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-010-0038-2