Abstract
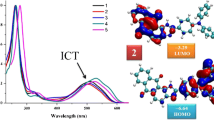
This study focuses on the preparation, single crystal X-ray diffraction, characterization, and optical properties of some anthraquinone-based dyes. The anthraquinone-based antimicrobial dye N-{2-[(9,10-dioxo-9,10-dihydroanthracen-1-yl)amino]-2-oxoethyl}-N,N-dimethylbutan-1-aminium chloride monohydrate (III) was obtained from 1-aminoanthraquinone (I) via 2-chloro-N-(9,10-dioxo-9,10-dihydroanthracen-1-yl)acetamide (II) using known preparation and characterization methods. Single crystal X-ray diffraction analysis of III revealed a monoclinic system, space group P21/n, Z = 4. Photoluminescence properties of anthraquinone dyes I–III were also investigated. These dyes gave an intense emission (λmax = 341 nm) upon the irradiation by UV light and showed photoluminescence quantum yields of 73 %, 66 %, and 61 % with long excited-state lifetimes of 6.87 ns, 6.14 ns, and 5.69 ns, respectively. These anthraquinone dyes are of interest as an organic light emitting material for electroluminescent devices.
Similar content being viewed by others
References
Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G., & Taylor, R. (1987). Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. Journal of Chemistry Society, Perkin Transaction 2, 1987, 1–19. DOI: 10.1039/P298700000S1.
Aslantas, M., Kurtoglu, N., Sahin, E., & Kurtoglu, M. (2007). 4-[(E)-Phenyldiazenyl]-2-[(E)-phenyliminomethyl]phenol. Acta Crystallographica Section E, E63, o3637. DOI: 10.1107/S1600536807036197.
Balabanova, M., Popova, L., & Tchipeva, R. (2003). Dyes in dermatology. Clinics in Dermatology, 21, 2–6. DOI:10.1016/S0738-081X(02)00330-9.
Berghot, M. A., & Moawad, E. B. (1995). Synthesis of novel substituted anthra[1,9-bc]pyridin-6-ones. Bollettino Chimico Farmaceutico, 134, 564–568.
Birbiçer, N., Kurtoğlu, M., & Serin, S. (1999). Synthesis of two new azo ligands containing polyoxyethylene glycol and their complexes with Ni(II), Cu(II) and Co(II). Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 29, 1353–1364. DOI: 10.1080/00945719909351704.
Boonnak, N., Chantrapromma, S., Fun, H. K., Anjum, S., Ali, S., Atta-ur-Rahman, & Karalai, C. (2005). 3-(3,7-Dimethylocta-2,6-dienyloxy)-1,8-dihydroxy-6-methyl-9,10-anthraquinone. Acta Crystallographica Section E, E61, o410–o412. DOI: 10.1107/S1600536805001674.
Chen, T. R., Chen, J. D., Keng, T. C., & Wang, J. C. (2001). A new pyridylamine for blue light electroluminescent devices. Tetrahedron Letter, 42, 7915–7917. DOI: 10.1016/S0040-4039(01)01693-8.
Dahiya, P., Choudhury, S. D., Maity, D. K., Mukherjee, T., & Pal, H. (2008). Solvent polarity induced structural changes in 2,6-diamino-9,10-anthraquinone dye. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 69, 134–141. DOI: 10.1016/j.saa.2007.03.018.
Farrugia, L. J. (1997). ORTEP-3 for Windows — a version of ORTEP-III with a graphical user interface (GUI). Journal of Applied Crystallography, 30, 565. DOI: 10.1107/S0021889897003117.
Gilbert, A., & Baggott, J. (1991). Essentials of molecular photochemistry. Boca Raton, FL, USA: CRC Press.
Gordon, P. F., & Gregory, P. (1983). Organic chemistry in colour (p. 162). Berlin, Germany: Springer.
Gregory, P. (1991). High-technology applications of organic colorants. New York, NY, USA: Plenum Press.
Guilbault, G. G. (Ed.) (1990). Practical fluorescence (2nd ed.). New York, NY, USA: Marcel Dekker.
Kirk, R. E., & Othmer, D. F. (1993). Dyes, anthraquinones. In J. I. Kroschwitz, & M. Howe-Grant (Eds.), Kirk-Othmer encyclopedia of chemical technology (4th ed., Vol. 4, pp. 653–666). New York, NY, USA: Wiley.
Kocaokutgen, H., Gür, M., Soylu, M. S., & Lönnecke, P. (2005). Spectroscopic, thermal and crystal structure properties of novel (E)-2,6-dimethyl-4-(4-tert-butylphenyldiazenyl)phenyl acrylate dye. Dyes and Pigments, 67, 99–103. DOI: 10.1016/j.dyepig.2004.09.021.
Kulkarni, A. P., Zhu, Y., & Jenekhe, S. A. (2005). Quinoxalinecontaining polyfluorenes: Synthesis, photophysics, and stable blue electroluminescence. Macromolecules, 38, 1553–1363. DOI: 10.1021/ma048118d.
Kurtoğlu, M., & Baydemir, S. A. (2007). Studies on mononuclear transition metal chelates derived from a novel (E, E)-dioxime: synthesis, characterization and biological activity. Journal of Coordination Chemistry, 60, 655–665. DOI:10.1080/00958970600896076.
Kurtoğlu, M., Birbiçer, N., Kimyonen, Ü., & Serin, S. (1999a). Determination of pKa values of some azo dyes in acetonitrile with perchloric acid. Dyes and Pigments, 41, 143–147. DOI: 10.1016/S0143-7208(98)00077-1.
Kurtoğlu, M., İspir, E., Kurtoğlu, N., & Serin, S. (2008). Novel vic-dioximes: Synthesis, complexation with transition metal ions, spectral studies and biological activity. Dyes and Pigments, 77, 75–80. DOI: 10.1016/j.dyepig.2007.03.010.
Kurtoğlu, N., Kurtoğlu, M., & Serin, S. (1999b). Synthesis and characterisation of some novel Schiff base metal complexes with polyoxyethylene glycols as substituents. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 29, 1779–1791. DOI: 10.1080/00945719909351735.
Leadbetter, P. W., & Leaver, A. T. (1989). Disperse dyes — the challenge of the 1990s. Review of Progress in Coloration, 19, 33–39.
Liu, J., & Sun, G. (2008). The synthesis of novel cationic anthraquinone dyes with high potent antimicrobial activity. Dyes and Pigments, 77, 380–386. DOI: 10.1016/j.dyepig.2007.06.009.
Ma, M., Sun, Y., & Sun, G. (2003). Antimicrobial cationic dyes: part 1: synthesis and characterization. Dyes and Pigments, 58, 27–35. DOI: 10.1016/S0143-7208(03)00025-1.
Malone, J. F., Andrews, S. J., Bullock, J. F., & Docherty, R. (1996). The solid state structure of CI Disperse Orange 44. Dyes and Pigments, 30, 183–200. DOI: 10.1016/0143-7208(95)00076-3.
Martelli, S., Dzieduszycka, M., Stefanska, B., Gracz, M. B., & Borowski, E. (1988). Synthesis and antineoplastic evaluations of 1,4-bis(aminoalkanamido)-9,10-anthracenediones. Journal of Medicinal Chemistry, 31, 1956–1959. DOI: 10.1021/jm00118a015.
Nardelli, M. (1995). PARST95 — an update to PARST: a system of Fortran routines for calculating molecular structure parameters from the results of crystal structure analyses. Journal of Applied Crystallography, 28, 659. DOI: 10.1107/S0021889895007138.
Øllgaard, H., Frost, L., Galster, J., & Hansen, O. C. (1999). Survey of azo-colorants in Denmark: Consumption, use, health and environmental aspects. Copenhagen: Ministry of Environment and Energy, Danish Environmental Protection Agency. (Miljøprojekt No. 509, No. XX 1998, pp. 147–290).
Osaheni, J. A., & Jenekhe, S. A. (1994). Enhancement of luminescence in polymer nanocomposites. Chemistry Materials, 6, 1906–1909. DOI: 10.1021/cm00047a002.
Patrauchan, M. A., & Oriel, P. J. (2003). Degradation of benzyldimethylalkylammonium chloride by Aeromonas hydrophila sp. K. Journal of Applied Microbiology, 94, 266–272. DOI: 10.1046/j.1365-2672.2003.01829.x.
Peters, A. T., & Freeman, H. S. (Eds.) (1991). Colour chemistry: The design and synthesis of organic dyes and pigments (pp. 193–195). Barking, UK: Elsevier.
Pu, Y. J., Kurata, T., Soma, M., Kido, J., & Nishide, H. (2004). Triphenylamine- and oxadiazole-substituted poly(1,4-phenylenevinylene) s: synthesis, photo-, and electroluminescent properties. Synthetic Metals, 143, 207–214. DOI: 10.1016/j.synthmet.2003.12.003.
Rembold, M. W., & Kramer, H. E. A. (1980). The role of anthraquinonoid dyes in the catalytic fading’of dye mixtures — substituent-dependent triplet yield of diaminoanthraquinones. Journal of the Society Dyers and Colourists, 96, 122–126. DOI: 10.1111/j.1478-4408.1980.tb03518.x.
Rigaku/MSC. (2005). CrystalClear [computer software]. Rigaku/MSC Inc.: The Woodlands, TX, USA.
Sadeghi-Aliabadi, H., Tabarzadi, M., & Zarghi, A. (2004). Synthesis and cytotoxic evaluation of two novel anthraquinone derivatives. Il Farmaco, 59, 645–649. DOI: 10.1016/j.farmac.2004.03.006.
Serin, S., & Kurtoğlu, M. (1994). Potentiometric titrations of some azo dyes containing a hydroxy group with tetrabutylammonium hydroxide in acetonitrile. Analyst, 119, 2213–2215. DOI: 10.1039/AN9941902213.
Sheldrick, G. M. (1997a). SHELXS-97. Program for crystal structure solution. Göttingen, Germany: University of Göttingen.
Sheldrick, G. M. (1997b). SHELXL-97. Program for refinement of crystal structures. Göttingen, Germany: University of Göttingen.
Shinar, J. (Ed.) (2004). Organic light-emitting devices: A survey. New York, NY, USA: Springer.
Singh, K., Mahajan, A., & Robinson, W. T. (2007). Single crystal X-ray structure analysis of some heterocyclic monoazo disperse dyes. Dyes and Pigments, 74, 95–102. DOI:10.1016/j.dyepig.2006.01.018.
Spek, A. L. (2003). PLATON, a multipurpose crystallographic tool. Utrecht, The Netherlands: University of Utrecht.
Sun, G., & Ma, M. (2005). U.S. Patent Application No. US 2005/0011012 A1. Arlington, VA, USA: United States Patent and Trademark Office.
Temel, A., Özbey, S., & Ertan, N. (1996). Crystal structure of hydrazone form of 1-butyl-3-cyano-6-hydroxy-4-methyl-5-(2-thiazolylazo)-2-(1H)-pyridone. Dyes and Pigments, 32, 237–244. DOI: 10.1016/S0143-7208(96)00028-9.
Wilson, A. J. C. (Ed.) (1995). International tables for crystallography: Mathematical, physical and chemical tables (2nd ed., Vol. C). Dordrecht, The Netherlands: Kluwer.
Yang, W., You, X. L., Zhong, Y., & Zhang, D. C. (2007). The crystal structure of 4-[(2-methoxy-4-nitro-phenylazo)-phenyl]-dimethyl-amine. Dyes and Pigments, 73, 317–321. DOI: 10.1016/j.dyepig.2005.11.013.
Zhao, D. X., Li, W. L., Hong, Z. R., Liu, X., Liang, C., & Zhao, D. (1999). White light emitting organic electroluminescent devices using lanthanide dinuclear complexes. Journal of Luminescence, 82, 105–109. DOI: 10.1016/S0022-2313(99)00038-1.
Zhu, J. C., Liang, Y., Wang, H. S., Pan, Y. M., & Zhang, Y. (2007). 1,3,8-Trihydroxy-6-methylanthraquinone monohydrate. Acta Crystallographica Section E, E63, 233–235. DOI: 10.1107/S1600536806049178.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurtoglu, N., Aslantaş, M., Zengin, H. et al. Single crystal X-ray structure and optical properties of anthraquinone-based dyes. Chem. Pap. 64, 645–651 (2010). https://doi.org/10.2478/s11696-010-0037-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-010-0037-3