Abstract
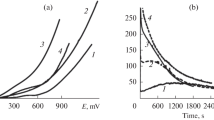
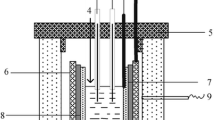
The anodic behaviour of silver was investigated in cryolite—alumina-based melt. Silver has a lower melting point (ca. 960°C) than the other metals considered as possible inert materials for aluminium electrolysis. The working temperature used in aluminium industry is approximately 960°C, depending on the melt composition. Therefore, the stability of silver during the anodic process was tested at 870°C in an acidic electrolyte consisting of 65.5 mass % Na3AlF6 + 22.9 mass % AlF3 + 5.7 mass % CaF2 + 3.9 mass % LiF + 2 mass % Al2O3 with the melting point ca. 850°C. The electrolyte without alumina was prepared as well, with the melting point ca. 860°C. The resulting cryolite ratio (CR = n(NaF)/n(AlF3)) for both electrolytes was equal to 1.6. The behaviour of the silver anode was studied by voltammetry measurements. The electrochemical study showed that an oxidation reaction occurred at a potential below the oxygen evolution potential. Silver was not found to be stable under oxygen evolution. The degradation of the silver anode was apparent after electrolysis.
Similar content being viewed by others
References
Thonstad, J., Fellner, P., Haarberg, G. M., Híveš, J., Kvande, H., and Sterten, Å., Aluminium Electrolysis. Fundamentals of the Hall-Héroult Process, 3rd Edition. Aluminium-Verlag, Düsseldorf, 2001.
Lorentsen, O. A., Thesis, The Norwegian University of Science and Technology, Trondheim, 2000.
Belyaev, A. I. and Studentsov, Ya. E., Legkie Metally 5, 15 (1936).
Belyaev, A. I. and Studentsov, Ya. E., Legkie Metally 6, 17 (1937).
Belyaev, A. I. and Studentsov, Ya. E., Legkie Metally 8, 7 (1938).
Ray, S. P. and Woods, R. W., U.S. 5,794,112 (1998).
Ray, S. P., Woods, R. W., Dawless, R. K., and Hosler, R. B., U.S. 5,865,980 (1999).
Ray, S. P., Woods, R. W., Dawless, R. K., Hosler, R. B., U.S. 6,126,799 (2000).
Ray, S. P., Liu, X., and Weirauch, D. A., Jr., U.S. 6,217,739 (2001).
Ray, S. P., Woods, R. W., Dawless, R. K., and Hosler, R. B., U.S. 6,332,969 (2001).
Ray, S. P., Liu, X., and Weirauch, D. A., Jr., U.S. 6,372,119 (2002).
Ray, S. P., Weirauch, D. A., Jr., and Liu, X., U.S. 6,423,195 (2002).
Chamelot, P., Lafage, B., and Taxil, P., J. Electrochem. Soc. 143, 1570 (1996).
Haupin, W. E., J. Metals 23, 46 (1971).
Solheim, A., Rolseth, S., Skybakmoen, E., Støen, L., Sterten, Å., and Støre, T., in Proceedings of the 124th TMS Annual Meeting, p. 451, Las Vegas, Nevada, 1995.
Barin, I., Knacke, O., and Kubaschewski, O., Thermochemical Properties of Inorganic Substances. Springer-Verlag, Berlin, 1991.
Cassayre, L., Chamelot, P., Arurault, L., and Taxil, P., J. Appl. Electrochem. 35, 999 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kucharík, M., Chamelot, P., Cassayre, L. et al. Silver as anode in cryolite—alumina-based melts. Chem. Pap. 61, 142–145 (2007). https://doi.org/10.2478/s11696-007-0011-x
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.2478/s11696-007-0011-x