Abstract
Introduction
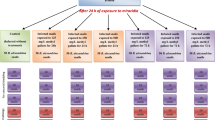
Pomacea lineata acts as the natural biological controller of Biomphalaria glabrata, the intermediate host of Schistosoma mansoni, as they are found in the same environment. However, there are no studies reporting an infection in P. lineata due to S. mansoni. Thus, this work investigated parameters related to the immunity of P. lineata after exposure for 24 and 48 h to S. mansoni under experimental conditions.
Methods
The F1 generation of these snails was used in this study. The total and differential counts of hemocytes, phenoloxidase, nitric oxide, total proteins, expression of TNF-α in hemocytes and histopathology of the head-foot organ were analyzed.
Results
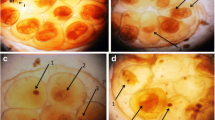
Exposure to S. mansoni promoted an increase in the total number of hemocytes, an increase of granulocytes, a reduction of agranulocytes and hyalinocytes, an increase in phenoloxidase levels, total proteins and nitric oxide. There was TNF-α expression in the agranulocytes and granulocytes, increasing in intensity after exposure to the trematode. Head-foot histopathology revealed the presence of sporocytes in the fibromuscular layer surrounded by granulation tissue only within 24 h. At 48 h, there was marked fibrosis in this layer and little granulation tissue.
Conclusion
Thus, we can conclude that P. lineata seems to trigger a series of immunological strategies in a very effective way that confers some resistance to S. mansoni.






Similar content being viewed by others
References
Abe, AS.1995. Estivation in South American amphibians and reptiles. Brazilian Journal of Medical and Biological Research, 28, 1241–1247.
Abdel-Halim K, El-Saad AA, Talha M, Hussein A, Bakry N. 2013. Oxidative stress on land snail Helix aspersa as a sentinel organism for ecotoxicological effects of urban pollution with heavy metals. Chemosphere, 93, 1131-1138. https://doi.org/10.1016/j.chemosphere.2013.06.042
Abou-El-Naga IF, Radwan, EH. 2012. Defense response of susceptible and resistant Biomphalaria alexandrina snails against Schistosoma mansoni infection. Revista de Biologia Tropical, 60 Suppl 3,1195-1204. https://doi.org/10.1590/s0074-02762010000700007
Allegretti SM, Carvalho JF, Magalhães LA, Zanotti-Magalhães EM. 2009. Behaviour of albino and melanic variants of Biomphalaria glabrata Say, 1818 (Mollusca: Planorbidae) following infection by Schistosoma mansoni Sambon, 1907. Brazilian Journal of Biology, 69 Suppl 1, 217-222. https://doi.org/10.1590/s1519-69842009000100029
Barboza SHR, Costa DPS, Romanelli PF. 2006. Processamento e avaliação sensorial da carne dos moluscos Escargot (Achatina fulica) e Aruá (Pomacea lineata). Alimentos e Nutrição Araraquara, 17, 413-418.
Barraco MA, Steil AA, Gargioni R. 1993. Morphological characterization of the hemocytes of the pulmonate snail Biomphalaria tenagophila. Memórias do Instituto Oswaldo Cruz, 88, 73-83. https://doi.org/10.1590/s0074-02761993000100012
Beschin A, Bilej M, Lucas, R, De Baetselier, P. 2003. Functional convergence of invertebrate and vertebrate cytokine-like molecules based on similar lectin-like activity. In Invertebrate Cytokines and the Phylogeny of Immunity: Facts and Paradoxes (Beschin, A. and Müller, W.E.G., eds.). Progress in Molecular and Subcellular Biology Series 145–163.
Bradford MM. 1976. A rapid and sensitive methods for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brayner FA, Araújo HRC, Cavalcanti MGS, Alves LC, Peixoto CA. 2005. Ultrastructural characterization of the hemocytes of Culex quinquefasciatus (Diptera: Culicidae). Micron, 36, 359-367. https://doi.org/10.1016/j.micron.2004.11.007
Brayner F, Araújo HRC, Santos SS, Cavalcanti MGS, Alves LC, Souza JRB, et al. 2007. Haemocyte population and ultrastructural changes during the immune response of the mosquito Culex quinquefasciatus to microfilariae of Wuchereria bancrofti. Medical and Veterinary Entomology., 21, 112-120. https://doi.org/10.1111/j.1365-2915.2007.00673.x
Cavalcanti MG, Filho FC, Mendonça AM, Duarte G R, Barbosa CC, Castro CM, Alves LC, Brayner FA. 2012. Morphological characterization of hemocytes from Biomphalaria glabrata and Biomphalaria straminea. Micron. 43:285–291.
Cazzaniga NJ. 1990. Predation of Pomacea canaliculata (Ampullariidae) on adult Biomphalaria peregrina (Planorbidae). Annals of Tropical Medicine & Parasitology., 84, 97–100. https://doi.org/10.1080/00034983.1990.11812439
Cerenius L, Soderhall K. 2004. The prophenoloxidase-activating system in invertebrates. Immunological Reviews, 198, 116-26. https://doi.org/10.1111/j.0105-2896.2004.00116.x
Chen C, Durrant HJ, Newton RP, Ratcliffe NA. 1995. A study of novel lectins and their involvement in the activation of the prophenoloxidase system in Blaberus discoidalis. Journal of Biochemistry, 310, 23-31.
Cimerman B, Cimerman S. 2005. Parasitologia Humana e seus fundamentos gerais. Atheneu, São Paulo.
Coustau C, Gourbal B, Duval D, Yoshino TP, Adema CM, Mitta G. 2015. Advances in gastropod immunity from the study of the interaction between the snail Biomphalaria glabrata and its parasites: A review of research progress over the last decade. Fish and Shellfish Immunology, 46, Suppl 1, 5-16. https://doi.org/10.1016/j.fsi.2015.01.036
Cueto JA, Vega IA, Castro-Vazquez A. 2013. Multicellular spheroid formation and evolutionary conserved behaviors of apple snail hemocytes in culture. Fish and Shellfish Immunology, 34, 443–453. https://doi.org/10.1016/j.fsi.2012.11.035
Cueto JA, Rodriguez C, Vega IA, Castro-Vazquez A. 2015. Immune Defenses of the Invasive Apple Snail Pomacea canaliculata (Caenogastropoda, Ampullariidae): Phagocytic hemocytes in the circulation and the kidney. Plos One. https://doi.org/10.1371/journal.pone.0123964
Das S, Khangarot BS. 2010. Efeitos do cobre sobre o desenvolvimento de ovos e eclosão de um caracol de água doce pulmonate Lymnaea luteola. Journal Hazardous Materials, 179, 665-675.
Dunn PE. 1986. Biochemical aspects of insect immunity. Annual Review. Entomology, 31, 321-339. https://doi.org/10.1146/annurev.en.31.010186.001541
Engels D, Chitsulo L, Montresor A, Savioli L. 2002. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Tropica, 82, 139-46. https://doi.org/10.1016/s0001-706x(02)00045-1
Faraldo AC, Nóbile PM, Daffre S, Gregório EA, Lello E. 2006. Prophenoloxidase activation in blowfly hemolymph after yeast inoculation. Anais do XIII Congresso da Sociedade Brasileira de Biologia Celular 136.
Ferreira UM, Foronda AS, Schumaker TTS. 2003. Fundamentos biológicos da parasitologia humana. Manole, São Paulo.
Galinier R, Portela J, Moné Y, Allienne JF, Henri H, Delbecq S, et al. 2013. Biomphalysin, a New b Pore-forming Toxin Involved in Biomphalaria glabrata Immune Defense against Schistosoma mansoni. PLoS pathogens, San Francisco 9, Suppl 3, 1–16. https://doi.org/10.1371/journal.ppat.1003216
Guaraldo AMA, Magalhães LA, Rangel HA, Pareja G. 1981. Evolução dos esporocistos de Schistosoma mansoni (Sambon, 1907) em Biomphalaria glabrata (Say, 1818) e Biomphalaria tenagophila (D’Orbigny, 1835). Revista de Saúde Pública, 15, 436-448. https://doi.org/10.1590/s0034-89101981000400008
Green LC, De Luzuriaga KR, Wagner DA, Rand W, Istfan N, Young VR et al. 1981. Nitrate biosynthesis in man. Proceedings of the National Academy of Scienses, 78, 7764–7768.
Hahn UK, Bender RC, Bayne CJ. 2000. Production of reactive oxygen species by hemocytes of Biomphalaria glabrata: Carbohydrate-specific stimulation. Developmental & Comparative Immunology, 24, 531-541. https://doi.org/10.1016/s0145-305x(00)00017-3
Hahn UK, Bender RC, Bayne CJ. 2001. Involvement of nitric oxide in killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata. Journal of Parasitology, 187, 778-785. https://doi.org/10.1645/0022-3395(2001)087%5b0778:ionoik%5d2.0.co;2
Hine PM. 1999. The inter-relationships of bivalve hemocytes. Fish and Shelfish Immunology, 9, 367-385. https://doi.org/10.1006/fsim.1998.0205
Igaki T, Kanda H, Yamamoto-Goto Y, Kanuka H, Kuranga E, Agaki T, et al. 2002. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. The Embo Journal, 21, 3009–3018. https://doi.org/10.1093/emboj/cdf306
Itziou A, Dimitriadis VK. 2011. Introduction of the land snail Eobania vermiculata as a bioindicator organism of terrestrial pollution using a battery of biomarkers. Science of The Total Environmen, 409, 1181-1192. https://doi.org/10.1016/j.scitotenv.2010.12.009
Jiravanichpaisal P, Lee BL, Söderhäll K. 2006. Cell-Mediated Immunity In arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology, 211, Suppl 4, 213-236. https://doi.org/10.1016/j.imbio.2005.10.015
Lee ES, Kim JH, Im S, Lee KB, Sonhn S, Kang WH. 2001. Application of computerized image analysis in pigmentary skin diseases. The International Journal of Dermatology, 40, 45-49. https://doi.org/10.1046/j.1365-4362.2001.00084.x
Lingpeng D, Wang W, Dong X, Hu R, Nan X. 2011. Molluscicidal activty of cardiac glycosides from Nerium indicum against Pomacea canaliculata and its implications for the mechanisms of toxicity. Environmental Toxicology and Pharmacology, 32, Suppl 2, 226-232. https://doi.org/10.1016/j.etap.2011.05.007
Loker ES. 2010. Gastropod immunobiology. In Soderhall K (ed) (AUSTIN: Landes Bioscience and Springer Science + Business Media), 17-43.
Machado SMP, Magalhães LA, Artigas PT, Cordeiro NS, Carvalho JF. 1988 Verificação de antagonismo entre larvas de Schistosoma mansoni e larvas e outros Digenea em Biomphalaria tenagophila molusco planorbídeo de criadouro natural situado na região de Campinas, SP, Brasil. Revista de Saúde Pública, 22, 484-488.
Marques MM, Barbosa F. 2000. Na fauna do fundo, o retrato da degradação. Ciência Hoje, 30, Suppl 175, 72-75.
Martin GG, Oakes CT, Tousignant HR. 2007. Structure and function of haemocytes in two marine gastropods, Megathura crenulata and Aplysia californica. Journal of Molluscan Studies, 73, 355-365. https://doi.org/10.1093/mollus/eym032
Matricon-Gondran M, Letocart M. 1999. Internal defenses of the snail Biomphalaria glabrata. I. Characterization of hemocytes and fixed phagocytes. Journal of Invertebrate Pathology, 74, 224–234. https://doi.org/10.1006/jipa.1999.4876
Mattos ACA. 2011. Estudo do sistema inato de defesa de Biomphalaria tenagophila (d’ Orbigny, 1835) frente ao Schistosoma mansoni Sambon, 1907. Dissertation, FIOCRUZ PE.
Melo, ES. 2015. Análise da variabilidade imunológica e da expressão dos genes FREPs entre as espécies Biomphalaria glabrata e B. straminea com diferentes perfis de suscetibilidade, frente à infecção por Schistosoma mansoni. Dissertation, FIOCRUZ PE.
Milward-de-Andrade R. 1978. Tentativa de colonização de lagoas do “Quadrilátero Ferrífero” (Nova Lima. MG. Brasil) com Pomacea haustrum (Reeve. 1856). In: Congresso da Sociedade Brasileira de Medicina Tropical, 14º Congresso da Sociedade Brasileira de Parasitologia, João Pessoa: p. 161.
Mitta G, Galinier R, Tisseyre P, Allienne JF, Girerd-Chambaz Y, Guillou F, et al. 2005. Gene discovery and expression analysis of immune-relevant genes from Biomphalaria glabrata hemocytes. Developmental and comparative immunology, 29 Suppl 5, 393–407. https://doi.org/10.1016/j.dci.2004.10.002
Moreno E, Yan M., Basler K. 2002. Evolution of TNF signalling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Current Biology, 12, 1263–1268. https://doi.org/10.1016/s0960-9822(02)00954-5
Nacif-Pimenta R, Mattos ACA, Orfano AS, Barbosa L, Pimenta PFP, Coelho PMZ. 2012. Schistosoma mansoni in Susceptible and Resistant Snail Strains Biomphalaria tenagophila: In Vivo Tissue Response and In Vitro Hemocyte Interactions. Plos One, 7, e45637 1-12. https://doi.org/10.1371/journal.pone.0045637
Negrão-Corrêa, D, Mattos ACA, Pereira CAJ, Martins-Souza RL. 2012. Interaction of Schistosoma mansoni Sporocysts and Hemocytes of Biomphalaria. Journal Parasitology Research, 1-6. https://doi.org/10.1155/2012/743920
Neves DP. 2004. Parasitologia Humana. Atheneu, São Paulo.
Newton WL. 1952. The comparative tissue reaction of two strains of Australorbis glabratus to infection with Schistosoma mansoni. Journal of Parasitology, 38, 362-6. https://doi.org/10.2307/3273773
Newton WL. 1953. The inheritance of suscetibility to infection with Schistosoma mansoni in Australorbis glabratus. Experimental Parasitology, 2, 242-57. https://doi.org/10.1016/0014-4894(53)90036-8
Oberholzer M, Östreicher M, Heinz C, Brühlmann. 1996. Métodos na análise quantitativa de imagens. Histoquímica e biologia celular. 105, 333-355.
Oliveira ALD, Levada PM, Zanotti-Magalhaes EM, Magalhães LA, Ribeiro-Paes JT. 2010. Differences in the number of hemocytes in the snail host Biomphalaria tenagophila, resistant and susceptible to Schistosoma mansoni infection. Genetics and Molecular Research, 9, 2436-2445. https://doi.org/10.4238/vol9-4gmr1143
Oliveira IHR. 2015. Identificação das proteínas diferencialmente expressas nos homécitos de populações de Biomphalaria tenagophila (Orbigny, 1835) (Gastropoda: Planorbidae) suscetível e resistentes a Schistosoma mansoni Sambon 1907 (Trematoda: Schistosomatidae). Dissertation, Universidade Federal de Minas Gerais.
Paulini, HM, Paulini E. 1971. Observações de laboratório sobre controle biológico de Biomphalaria glabrata pela Pomacea sp.(Ampullariidae). Revista Brasileira de Malariologia e Doenças Tropicais, 23,135-149.
Pinder AW, Storey KB, Ultsch GR. 1992. Aestivation and hibernation. In: Burggren, WW (ed), Environmental Biology of the Amphibia, University of Chicago Press, Chicago, pp 250–274.
Pipe RK. 1992. Generation of reactive oxygen metabolites by the hemocytes of the mussel Mytilus edulis. Developmental and Comparative Immunology, 16, 111-122. https://doi.org/10.1016/0145-305x(92)90012-2
Pointier JP, Giboda M. 1999. The case for biological control of snail intermediate hosts of Schistosoma mansoni. Parasitology Today, 15, 395–397. https://doi.org/10.1016/s0169-4758(99)01517-3
Pointier JP, Jourdane J. 2000. Biological control of the snail hosts of schistosomiasis in areas of low transmission: the example of the Caribbean area. Acta Tropica, 77, 53–60. https://doi.org/10.1016/s0001-706x(00)00123-6
Portela-Junior NC. 2016. Análise proteômica da hemolinfa de Biomphalaria glabrata, Biomphalaria straminea e Biomphalaria straminea R3 frente à exposição ao Schistosoma mansoni. Dissertation, FIOCRUZ PE.
Quaresma JA, Lima LW, Fuzii HT, Libonati RM, Pagliari C, Duarte MI. 2010. Immunohistochemical evaluation of macrophage activity and its relationship with apoptotic cell death in the polar forms of leprosy. Microbial Pathogenesis, 49, 135-40. https://doi.org/10.1016/j.micpath.2010.05.003
Ratcliffe NA, Rowley AF, Fitzgerald SW, Rhodes CP. 1985. Invertebrate imunity: Basic concepts and recent advances. International Review of Cytology, 97,183-350. https://doi.org/10.1016/s0074-7696(08)62351-7
Richards CS. 1970. Genetic of a molluscan vector of schistosomiasis. Nature, 227, 806-810. https://doi.org/10.1038/227806a0
Richards CS. 1977. Schistosoma mansoni: susceptibility reversal with age in the snail host Biomphalaria glabrata. Experimental Parasitology, 42, 165-168. https://doi.org/10.1016/0014-4894(77)90074-1
Roger E, Mitta G, Moné Y, Bouchut A, Rognon A, Grunau C, et al. 2008 Molecular determinants of compatibility polymorphism in the Biomphalaria glabrata/Schistosoma mansoni model: new candidates identified by a global comparative proteomics approach. Molecular and Biochemical Parasitology, 157, 205-216. https://doi.org/10.1016/j.molbiopara.2007.11.003
Ruppert EE, Fox RS, Barnes RD. 2005. Zoologia dos Invertebrados. Editora Roca, São Paulo.
Santos MAV, Rodrigues IRC. 2006. Estudo in vitro da ação letal da hemolinfa de Biomphalaria glabrata sobre os miracídios de Schistosoma mansoni. Revista Paraense de Medicina, 20 Suppl 2, 13–16.
Santos DVV, Santos MAV, Rodrigues IRC. 2011. Hemocyte production in Biomphalaria glabrata snails after exposure to different Schistosoma mansoni infection protocols. Revista Pan-Amazônica de Saúde, 2 Suppl 2, 33-38. https://doi.org/10.5123/s2176-62232011000200005
Shozawa A, Suto C. 1990. Hemocytes of Pomacea canaliculata: I. Reversible aggregation induced by Ca2 + . Developmental And Comparative Immunology, 14, 175–184. https://doi.org/10.1016/0145-305x(90)90089-w
Simões E. 2011. Impacto da floração da alga nociva Dinophysis acuminata sobre o sistema imune de ostras Crassostrea gigas e mexilhões Perna perna cultivados em Santa Catarina. Dissertation, Universidade Federal de Santa Catarina.
Souza CP, Cunha RCP, Andrade ZA. 1995. Desenvolvimento do Schistosoma mansoni em Biomphalaria tenagophila, Biomphalaria straminea e Biomphalaria glabrata. Revista do Instituto de Medicina Tropical, 37, 201-206. https://doi.org/10.1590/s0036-46651995000300004
Souza CP, Borges CC, Santana AG, Andrade ZA. 1997. Comparative histopathology of Biomphalaria glabrata, B. tenagophila and B. straminea with variable degrees of resistance to Schistosoma mansoni miracidia. Memórias do Instituto Oswaldo Cruz, 92, 517-522. https://doi.org/10.1590/s0074-02761997000400014
Sullivan JT, Richards CS. 1981. Schistosoma mansoni, NIH-SM-PR-2 estirpe, em ações sensíveis e não sensíveis de Biomphalaria glabrata: histologia comparativa. Journal of Parasitology, 5, 702-708.
Thiengo S. 1995. Família Pilidae Connoly, 1927 (Ampulariidae Gray, 1824). In: BARBOSA, FS (org.) Tóp. Malacol. Méd; Editora Fiocruz, p. 50-69.
Tunholi VM, Lustrino D, Tunholi-Alves VM, Mello-Silva CC, Maldonado A Jr, Pinheiro J, et al. 2011. Biochemical profile of Biomphalaria glabrata (Mollusca: Gastropoda) after infection by Echinostoma paraensei (Trematoda: Echinostomatidae). Parasitology Research, 109, 885-891. https://doi.org/10.1007/s00436-011-2330-7
Van Der Knaap WP, Loker ES. 1990. Immune mechanisms in trematode snail interactions. Parasitology Today, 6, 175–182. https://doi.org/10.1016/0169-4758(90)90349-9
Vargas-Albores F, Barracco MA. 2001. Defense mechanisms in bivalve mollusks, with emphasis in pectinids. In: Maeda-Martínez, A. N. (Ed.). Los Moluscos Pectínidos de Iberoamérica: Ciencia y Acuicultura. Noriega Editores, México pp. 127–146.
WU, X, Cheng B, Cai Z-D, Lou L-M. 2013. Determination of the apoptotic index in osteosarcoma tissue and its relationship with patients prognosis. Cancer Cell International, 13, 1-4. https://doi.org/10.1186/1475-2867-13-56
Zahoor Z, Davies AJ, Kirk RS, Rollinson D, Walker AJ. 2009. Nitric oxide production by Biomphalaria glabrata haemocytes: effects of Schistosoma mansoni ESPs and regulation through the extracellular signal-regulated kinase pathway. Parasits & Vectors, 2, 18. https://doi.org/10.1186/1756-3305-2-18
Zelck UE, Becker W, Bayne CJ. 1995. The plasma proteins of Biomphalaria glabrata in the presence and absence of Schistosoma mansoni. Developmental And Comparative Immunology, 19, 181-194. https://doi.org/10.1016/0145-305x(95)00012-i
Zoysa M, Jung S, Lee J. 2009. First molluscan TNF-a homologue of the TNF superfamily in disk abalone: Molecular characterization and expression analysis. Fish and Shellfish Immunology, 26, 625–631. https://doi.org/10.1016/j.fsi.2008.10.004
Acknowledgements
To the Coordination of Improvement of Higher Education Personnel (CAPES) for the granting of the PhD scholarship, to the Aggeu Magalhães Research Center—Keiso Asami Immunopathology Laboratory of UFPE and to the Laboratories of Histology and Ecophysiology and Animal Behavior of UFRPE for having open its doors to the realization of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, B.B.T.N., Alves, L.C., Brayner, F.A. et al. Immunological Parameters of the Pomacea lineata Spix, 1827 (Mollusca: Caenogastropoda) Exposed to Schistosoma mansoni Sambon, 1907. Acta Parasit. 64, 31–43 (2019). https://doi.org/10.2478/s11686-018-00005-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-018-00005-9