Abstract
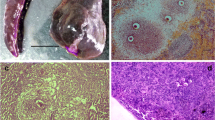
The ultrastructure of gamonts and sporulated oocysts of Goussia metchnikovi in the spleen of gudgeon, Gobio gobio from the river Lee, England is described. In developing microgamonts, small amylopectin granules were grouped centrally and nuclei were often arranged peripherally, close to the surface membrane. Nuclear chromatin condensed into peripheral dense portions that became the nuclei of flagellated microgametes, released to the parasitophorous vacuole. The cytoplasm of macrogametes had larger, scattered amylopectin granules, lipid globules and small electron-dense bodies, but no obvious wall forming bodies; peripheral vesicular structures with the appearance of mitochondria were also present and the parasitophorous vacuole contained flocculent material, but was otherwise free of structures. Sporulated oocysts contained four sporocysts and oocyst walls appeared to consist of a single membrane. Sporocyst walls showed a dehiscence suture, characteristic of the genus Goussia, which had filamentous extensions in places. The sporocyst wall comprised a dense inner layer and a thin outer layer with a fuzzy coat, separated by an electron lucent layer. Groups of oocysts were encapsulated by fibrous layers and inflammatory cells, and many sporocysts and their contained sporozoites showed evidence of elimination by the host.
Similar content being viewed by others
References
Agius C., Agbede S.A. 1984. An electron microscopical study on the genesis of lipofuschin, melanin and haemosiderin in the haemopoietic tissues of fish. Journal of Fish Biology, 24, 471–488. DOI: 10.1111/j.1095-8649.1984.tb04818.x.
Agius C., Roberts R.J. 2003. Melano-macrophage centres and their role in fish pathology. Journal of Fish Diseases, 26, 499–509. DOI: 10.1046/j.1365-2761.2003.00485.x.
Ball S.J. 1983. Eimeria metchnikovi (Laveran, 1897) Reichenow, 1921 from the gudgeon, Gobio gobio L., in south east England. Journal of Fish Diseases, 6, 210–203. DOI: 10.1111/j.1365-2761.1983.tb00068.x.
Békési L., Molnár K. 1991. Calyptospora tucunarensis n. sp. (Apicomplexa: Sporozoea) from the liver of tucurae Cichia ocellaris in Brazil. Systematic Parasitology, 18, 127–132. DOI: 10.1007/BF00017665.
Belli S.I., Smith N.C., Ferguson D.J.P. 2006. The coccidian oocyst: a tough nut to crack! Trends in Parasitology, 22, 416–423. DOI: 10.1016/j.pt.2006.07.004.
Davies A.J. 1990. Ultrastructural observations on the endogenous stages of Eimeria variabilis (Thélohan, 1893) Reichenow, 1921, from Cottus (Taurulus) bubalis Euphrasen (Teleostei: Cottidae). Journal of Fish Diseases, 13, 447–461. DOI: 10.1111/j.1365-2761.1990.tb00804.x.
Davies A.J., Ball S.J. 1993. The biology of fish coccidia. Advances in Parasitology, 32, 293–366. DOI: 10.1016/S0065-308X(08)60210-9.
Davies A.J., Stewart B. 2000. Autofluorescence in the oocysts of marine and freshwater fish coccidia. Folia Parasitologica, 47, 157–158.
Desser S.S., Li L. 1984. Ultrastructural observations on the sexual stages and oocyst formation in Eimeria laureleus (Protozoa, Coccidia) of perch Perca flavescens, from Lake Sasejewun, Ontario. Zeitschrift für Parasitenkunde, 70, 153–164.
Duszynski D.W., Couch L., Upton S.J. 2000. Coccidia (Eimeriidae) of Cypriniformes (Cyprinids). Biology.unm.edu/biology/coccidia/cyprin.html supported by grant NSF grant PEET DEB 9521687.
Dyková I., Lom J. 1981. Fish coccidia: critical notes on life cycles, classification and pathogenicity. Journal of Fish Diseases, 4, 487–505. DOI: 10.1111/j.1365-2761.1981.tb01161.x.
Dyková I., Lom J. 1983. Fish coccidia: an annotated list of described species. Folia Parasitologica, 30, 193–208.
Dyková I., Lom J. 2007. Histopathology of Protistan and Myxozoan Infections in Fishes. An Atlas. Academia Praha, Czech Republic, pp. 1–219.
Gestal C., Azevedo C. 2006. Ultrastructural aspects of hepatic coccidiosis caused by Goussia lusca n. sp. (Apicomplexa: Coccidia) infecting Trisopterus luscus (Gadidae) from the NE Atlantic Ocean. Diseases of Aquatic Organisms, 71, 25–31. DOI: 10.3354/dao071025.
Gjurčević E., Kozarić Z., Bambir S., Petrinec Z., Kužir S., Gudan A., Baždarić B. 2008. Histological investigations of Eimeria infection in large-scaled gurnards, Lepidotrigla cavillone (Lacepède, 1801) from the Novigrad Sea, Croatia. Acta Parasitologica, 53, 81–84. DOI: 10.2478/s11686-008-0009-8.
Jirků M., Modry D., Šlapeta J.R., Koudela B., Lukeš J. 2002. The phylogeny of Goussia and Choleoeimeria (Apicomplexa; Eimeriorina) and the evolution of excystation structures in coccidian. Protist, 153, 379–390. DOI: 10.1078/14344610260450118.
Lom J. 1971. Remarks on the spore envelopes in fish coccidia. Folia Parasitologica, 18, 289–293.
Lom J., Desser S.S., Dyková I. 1989. Some little-known and new protozoan parasites of fish from Lake Sasajewun, Algonquin Park, Ontario. Canadian Journal of Zoology, 67, 1372–1379. DOI: 10.1139/z89-195.
Lom J., Dyková I. 1992. Protozoan Parasites of Fishes. In: Developments in Aquaculture and Fisheries Science, 26. Elsevier, Amsterdam, pp. 1–315.
Mai K., Sharman P.A., Walker R.A., Katrib M., De Souza D., Mc-Conville M.J., Wallach M.G., Belli S.I., Ferguson D.J.P., Smith N.C. 2009. Oocyst wall formation and composition in coccidian parasites. Memórias do Instituto Oswaldo Cruz, 104, 281–289. DOI: 10.1590/S0074-02762009000200022.
Molnár K. 1984. Some peculiarities of oocyst rejection of fish coccidia. Symposia Biologica Hungarica, 23, 87–97.
Odense P.H., Logan V.H. 1976. Prevalence and morphology of Eimeria gadi (Fiebiger, 1913) in the haddock. Journal of Protozoology, 23, 564–571. DOI: 10.1111/j.1550-7408.1976. tb03842.x.
Overstreet R., Hawkins W.E., Fournie J.W. 1984. The coccidian genus Calyptospora n.g. and family Calyptosporidae n.fam. (Apicomplexa), with members infecting primarily fishes. Journal of Protozoology, 31, 332–339. DOI: 10.1111/j.1550-7408.1984.tb02972.x.
Paterson W.B., Desser S.S. 1981. Ultrastructure of macrogametogenesis, macrogametes and young oocysts of Eimeria iroquoina Molnár and Fernando, 1974 in experimentally infected fathead minnows (Pimephales promelas, Cyprinidae). Journal of Parasitology, 67, 496–504.
Paterson W.B., Desser S.S. 1982. The biology of two Eimeria species (Protists: Apicomplexa) in their mutual fish hosts in Ontario. Canadian Journal of Zoology, 60, 764–775. DOI: 10.1139/z82-106.
Paterson W.B., Desser S.S. 1984. Ultrastructural observations on fertilization and sporulation in Goussia iroquoina (Molnar & Fernando, 1974) in experimentally infected fathead minnows (Pimephales promelas, Cyprinidae). Journal of Parasitology, 70, 703–711. DOI: 10.2307/3281753.
Pellérdy L., Molnár K. 1968. Known and unknown eimerian parasites of fishes in Hungary. Folia Parasitologica, 15, 97–105.
Pittilo R.M., Ball S.J. 1979. The fine structure of the developing macrogamete of Eimeria maxima. Parasitology, 79, 259–265. DOI: 10.1017/S0031182000053336.
Scholtyseck E. 1973. Ultrastructure. In: (Eds. D.M. Hammond and P.L. Long) The Coccidia: Eimeria, Isospora, Toxoplasma and related genera. University Park Press, Baltimore and Butterwork, London, pp. 81–144.
Scholtyseck E., Melhom H., Hammond D. 1971. Fine structure of macrogametes and oocysts of coccidia and related organisms. Zeitschrift für Parasitenkunde, 37, 1–43.
Steinhagen D., Stemmer B., Körting W. 1994. Goussia aculeati from the three-spined stickleback, (Gasterosteus aculeatus): field observations and ultrastructural features. Applied Parasitology, 35, 99–106.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ball, S.J., Daszak, P. & Davies, A.J. Utrastructural observations on Goussia metchnikovi (Laveran, 1897) in the spleen of gudgeon, Gobio gobio L.. Acta Parasit. 57, 20–25 (2012). https://doi.org/10.2478/s11686-012-0014-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-012-0014-9