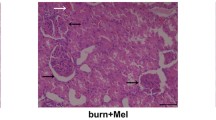
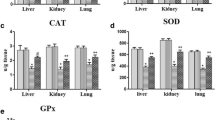
Abstract
Severe thermal injury may be complicated by dysfunction of organs distant from the original burn wound, including the liver, and represents a serious clinical problem. Although pathophysiology of burn-induced liver injury remains unclear, increasing evidence implicate activation of inflammatory response, oxidative stress, endothelial dysfunction and microcirculatory disorders as the main mechanisms of hepatic injury. Several studies suggest melatonin as a multifunctional indolamine that counteracts some of the pathophysiologic steps and displays significant beneficial effects against burn-induced cellular injury. This review summarizes the role of melatonin in restricting the burn-induced hepatic injury and focuses on its effects on oxidative stress, inflammatory response, endothelial dysfunction and microcirculatory disorders as well as on signaling pathways such as regulation of nuclear erythroid 2-related factor 2 (Nrf2) and nuclear factor-kappaB (NF-kB). Further studies are necessary to elucidate the modulating effect of melatonin on the transcription factor responsible for the regulation of the pro-inflammatory and antioxidant genes involved in burn injuries.
Similar content being viewed by others
Reference
Agay D., Andriollo-Sanchez M., Claeyssen R et al. Interleukin-6, TNF-alpha and interleukin-1 beta levels in blood and tissue in severely burned rats. Eur. Cytokine. Netw., 2008, 19(1), 1–7
Jeschke M.G., Chinkes D.L., Finnerty C.C et al. Pathophysiologic response to severe burn injury. Ann. Surg., 2008, 248(3), 387–401
Yang Q., Orman M.A., Berthiaume F et al. Dynamics ofshort-term gene expression profiling in liver following thermal injury. J. Surg. Res., 2012, 176(2), 549–558
Latha B., Babu M. The involvement of free radicals in burn injury: a review. Burns, 2001, 27, 309–317
Parihar A., Parihar M.S., Milner S., Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns, 2008, 34(1), 6–17
Korkmaz A., Reiter R.J., Topal T. et al. Melatonin: an established antioxidant worthy of use in clinical trials. Mol. Med, 2009, 15(1–2), 43–50
Rodriguez C., Mayo J.C., Sainz R.M.et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res, 2004, 36(1), 1–9
Maldonado M.D., Murillo-Cabezas F., Calvo J.R aet al. Melatonin as pharmacologic support in burn patients: a proposed solution to thermal injury-related lymphocytopenia and oxidative damage. Crit. Care Med, 2007,35, 1177–1185
Radogna F., Diederich M., Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochem Pharmacol., 2010, 80(12), 1844–1852
Mauriz J.L., Collado P.S., Veneroso C et al. A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J Pineal Res., 2012, May 31. doi:10.1111/j.1600-079X.2012.01014.x.
Jung K.H., Hong S.W., Zheng H.M., Lee D.H., Hong S.S. Melatonin downregulates nuclear erythroid 2-related factor 2 and nuclear factor-kappaB during prevention ofoxidative liver injury in a dimethylnitrosamine model. J Pineal Res., 2009,47(2), 173–183
El-Sokkary G.H., Abdel-Rahman G.H., Kamel E.S. Melatonin protects against lead-induced hepatic and renal toxicity in male rats. Toxicology., 2005, 213(1–2), 25–33
Chiu M.H., Su C.L., Chen C.F et al. Protective effect of melatonin on liver ischemia-reperfusion induced pulmonary microvascular injury in rats. Transplant Proc., 2012, 44(4), 962–965
Pintaudi A.M., Tesoriere L., D’Arpa N et al. Oxidative stress after moderate to extensive burning inhumans. Free Radic Res., 2000, 33(2), 139–146
Bekyarova G., Galunska B., Ivanova D., Yankova T. Effect of melatonin on burn-induced gastric mucosal injury in rats. Burns., 2009,35(6), 863–868
Sener G., Sehirli A.O., Satiroglu H et al. Melatonin improves oxidative organ damage in a rat model of thermal injury. Burns, 2002, 28, 419–425
Rawlingson A. Nitric oxide, inflammation and acute burn injury. Burns, 2003,29(7), 631–640
Bekiarova G.I., Markova M.P., Kagan V.G. [Alphatocopherol protection of erythrocytes from hemolysis induced by thermal injury]. Biull Eksp Biol Med., 1989,107(4), 413–415
Sandre C., Agay D., Ducros V et al. Early evolution of selenium status and oxidative stress parameters in rat models of thermal injury. J Trace Elem Med Biol., 2004,17(4), 313–318
Agay D., Anderson R.A., Sandre C et al. Alterations of antioxidant trace elements (Zn, Se, Cu) and related metallo-enzymes in plasma and tissues following burn injury in rats. Burns, 2005, 31(3), 366–371
Wang B.H., Yu X.J., Wang D et al. Alterations of traceelements (Zn, Se, Cu, Fe) and related metalloenzymes in rabbit blood after severe trauma. J Trace Elem Med Biol., 2007,21(2), 102–107
Ding H.Q., Zhou B.J., Liu L., Cheng S. Oxidative stress and metallothionein expression in the liver of rats with severe thermal injury. Burns, 2002,28(3), 215–121
Pascua P., Camello-Almaraz C., Camello P.J et al. Melatonin, and to a lesser extent growth hormone, restores colonic smooth muscle physiology in old rats. J Pineal Res., 2011,51(4), 405–415
Sener G., Kabasakal L., Cetinel S et al. Leukotriene receptor blocker montelukast protects against burn-induced oxidative injury of he skin and remote organs. Burns., 2005,31(5), 587–596
Bekyarova G., Tancheva S., Hristova M. Protective effect of melatonin against oxidative hepatic injury after experimental thermal trauma, Methods Find. Exp. Clin. Pharmacol., 2009, 31, 11–14
Bekyarova G., Apostolova M., Kotzev I. Melatonin protection against burn-induced hepatic injury by down-regulation of nuclear factor kappa B activation. Int J Immunopathol Pharmacol., 2012, 25(3), 591–596
Allegra M., Reiter R.J., Tan T.Xet al. The chemistry of melatonin’s interaction with reactive species. J Pineal Res, 2003,3, 1–10
Reiter R.J., Tan D.X., Manchester L.C., Manchester W.Qi, Karbownik M., Carlo J.R., et al. Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol Signals Recept, 2000, 9, 160–171
Antolín I., Rodríguez C., Saínz R.M et al. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. FASEB J., 1996,10(8), 882–890
Schaffazick S.R., Pohlmann A.R., de Cordova C.A et al. Protective properties of melatonin-loaded nanoparticles against lipid peroxidation. Int J Pharm., 2005,289(1–2), 209–213
Reiter R.J., Tan D.X., Sainz R.M et al. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol., 2002,54(10), 1299–321
Dahiya P. Burns as a model of SIRS. Front Biosci., 2009, 14, 4962–4967
Fang W.H., Yao Y.M., Shi Z.G et al. The mRNA expression patterns of tumor necrosis factor-alpha and TNFR-I in some vital organs after thermal injury. World J Gastroenterol., 2003,9(5), 1038–1044
Sun B.W., Sun Y., Sun Z.W., Chen X. CO liberated from CORM-2 modulates the inflammatory response in the liver of thermally injured mice. World J Gastroenterol., 2008, 14(4), 547–553
Işeri S.O., Düşünceli F., Erzik C et al. Oxytocin or social housing alleviates local burn injury in rats. J Surg Res., 2010,162(1), 122–131
Horton J.W. Free radicals and lipid peroxidation mediated injury in burn trauma: the role of antioxidant therapy. Toxicology., 2003,189(1–2), 75–88
Nishiura T., Nishimura T., deSerres S et al. Gene expression and cytokine and enzyme activation in the liver after a burn injury. J Burn Care Rehabil., 2000, 21(2), 35–141
Wullaert A., van Loo G., Heyninck K et al. Hepatic tumor necrosis factor signaling and nuclear factorkappaB: effects on liver homeostasis and beyond. Endocr Rev., 2007,28(4), 365–386
Ulrich D., Noah E.M., Pallua N. [Plasma endotoxin, procalcitonin, C-reactive protein, and organ functions in patients with major burns]. Handchir Mikrochir Plast Chir., 2001, 33(4), 262–266
Haider D.G., Leuchten N., Schaller G et al. C-reactive protein is expressed and secreted by peripheral blood mononuclear cells. Clin Exp Immunol., 2006,146(3), 533–539
Li J.Y., Yin H.Z., Gu X et al. Melatonin protects liver from intestine ischemia reperfusion injury in rats. World J Gastroenterol, 2008, 14, 7392–7396
Hu S., Yin S., Jiang X et al. Melatonin protects against alcoholic liver injury by attenuating oxidative stress, inflammatory response, and apoptosis. Eur J Pharmacol., 2009, 616(1–3), 287–92
Gravante G., Delogu D., Sconocchia G. “Systemic apoptotic response” after thermal burns. Apoptosis, 2007, 12, 259–270
Jeschke M.G., Low J.F., Spies M et al. Cell proliferation, apoptosis, NF-kappaB expression, enzyme, protein, and weight changes in livers of burned rats. Am J Physiol Gastrointest Liver Physiol., 2001, 280(6), G1314–320
Jaeschke H., Bajt M.L. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci., 2006, 89(1), 31–41
Muriel P. Role of free radicals in liver diseases. Hepatol Int, 2009, 3, 526–536
Acuña Castroviejo D., López L.C., Escames G et al. Melatonin mitochondria interplay in health and disease. Curr Top Med Chem., 2011,11(2), 221–240
Radogna F., Cristofanon S., Paternoster L et al. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J Pineal Res., 2008,44(3), 316–325
Lucken-Ardjomande S., Martinou J.C. Regulation of Bcl-2 proteins and of the permeability of the outer mitochondrial membrane. C R Biol., 2005, 328(7), 616–631
Cristofanon S., Uguccioni F., Cerella C et al. Intracellular prooxidant activity of melatonin induces a survival pathway involving NF kappaB activation. Ann N Y Acad Sci., 2009, 1171, 472–478
Zheng H., Chen X.L., Han Z.X et al. Effect of Ligustrazine on liver injury after burn trauma. Burns, 2006,32(3), 328–334
Barnes P.J., Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med., 1997,336(15), 1066–1071
Verma S., Badiwala M.V., Weisel R.D et al. C-reactive protein activates the nuclear factorkappaB signal transduction pathway in saphenous vein endothelial cells: implications for atherosclerosis and restenosis. J Thorac Cardiovasc Surg., 2003,126(6), 1886–1891
Li J.H., Yu J.P., Yu H.G et al. Melatonin reduces inflammatory injury through inhibiting NF-kappa B activation in rats with colitis. Mediators of Inflamm, 2005, 2005(4), 185–193
Sahib A.S., Al-Jawad F.H., Al-Kaisy A.A. Burns, endothelial dysfunction, and oxidative stress: the role of antioxidants. Ann Burns Fire Disasters., 2009, 22(1), 6–11
Lum H., Roebuck K.A.: Oxidative stress and endothelial cell dysfunction. Am. J. Physiol. Cell. Physiol., 2001, 280, C719–C41
Goligorsky M.S. Endothelial cell dysfunction: Can’t live with it, how to live without it. Am. J. Physiol. Renal. Physiol., 2005, 288, F871–880
Dhainaut J.F., Marin N., Mignon A., Vinsonneau C. Hepatic response to sepsis: Interaction between coagulation and inflammatory processes. Crit Care Med, 2001, 29(7), S2–47
Nan B., Yang H., Yan S et al. Creactive protein decreases expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Surgery, 2005, 138(2), 212–222
Bekyarova G., Tancheva S., Hristova M. The effects of melatonin on burn-induced inflammatory responses and coagulation disorders in rats. Methods Find Exp Clin Pharmacol., 2010, 32(5), 299–303
Tunali T., Sener G., Yarat A., Emekli N. Melatonin reduces oxidative damage to skin and normalizes blood coagulation in a rat model of thermal injury. Life Sci, 2005, 76(11), 1259–1265
Bekyarova G., Yankova T., Kozarev I., Yankov D. Reduced erythrocyte deformability related to activated lipid peroxidation during the early postburn period. Burns, 1996,22, 291–294
Bekyarova G. Relationship between enhanced platelet aggregation and oxidative alteration of erythrocytes in the early phase after thermal injury. Pathophysiology, 1998, 5,suppl. 1, 180–180
Levin G.Y., Egorihina M.N. The role of fibrinogen in aggregation of platelets in burn injury. Burns, 2010,36(6), 806–810
Park S.W., Choi S.M., Lee S.M. Effect of melatonin on altered expression of vasoregulatory genes during hepatic ischemia/reperfusion. Arch Pharm Res., 2007,30(12), 1619–1624
Lausevic Z., Lausevic M., Trbojevic-Stankovic J et al. Predicting multiple organ failure in patients with severe trauma. Can J Surg., 2008, 51(2), 97–102
Jeschke M.G. The hepatic response to thermal injury: is the liver important for postburn outcomes? Mol Med., 2009, 15(9–10), 337–351
Jeschke M.G., Micak R.P., Finnerty C.C., Herndon D.N. Changes in liver function and size after a severe thermal injury. Shock, 2007, 28(2), 172–177
Ryter S.W., Otterbein L.E., Morse D., Choi A.M. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem., 2002,234–235(1–2), 249–263
Devey L., Ferenbach D., Mohr E et al. Tissueresident macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism. Mol Ther., 2009, 17, 65–72
Kamimoto M., Mizuno S., Matsumoto K., Nakamura T. Hepatocyte growth factor prevents multiple organ injuries in endotoxemic mice through a heme oxygenase-1-dependent mechanism. Biochem Biophys Res Commun; 2009, 6,380, 333–337
Nakahira K., Takahashi T., Shimizu H et al. Protective role of heme oxygenase-1 induction in carbon tetrachloride-induced hepatotoxicity. Biochem Pharmacol., 2003, 66, 1091–1105
Nakae H., Inaba H. Expression of heme oxygenase-1 in the lung and liver tissues in a rat model of burns. Burns, 2002, 28(4), 305–309
Grochot-Przeczek A., Dulak J., Jozkowicz A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin Sci (Lond)., 201, 122(3), 93–103
Piantadosi C.A, Carraway M.S, Suliman H.B. Carbon monoxide, oxidative stress, and mitochondrial permeability pore transition. Free Radic Biol Med., 2006, 15, 1332–1339
Soares M.P., Seldon M.P., Gregoire I.P et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial activation. J Immunol., 2004, 172, 3553–3563
Sass G., Soares M.C., Yamashita K et al. Heme oxygenase-1 and its reaction product, carbon monoxide, prevent inflammation-related apoptotic liver damage in mice. Hepatology, 2003, 38, 909–918
Otterbein L. E., Bach F. H., Alam J et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med., 2000, 6, 422–428
Lee T.S., Chau L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med., 2002;8(3), 240–246
Morisaki H., Katayama T., Kotake Y et al. Carbon monoxide modulates endotoxin-induced microvascular leukocyte adhesion through platelet-dependent mechanisms. Anesthesiology, 2002, 97(3), 701–709
Durante W. Carbon monoxide and bile pigments: surprising mediators of vascular function. Vasc Med., 2002, 7(3), 195–202
Wunder C., Potter R.F. The heme oxygenase system: its role in liverinflammation. Curr Drug Targets Cardiovasc Haematol Disord., 2003, 3(3), 199–208
Searles C.D. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression. Am J Physiol Cell Physiol., 2006,291(5), C803–816
Bach F.H. Heme oxygenase-1 as a protective gene. Wien Klin Wochenschr., 2002,114Suppl 4, 1–3
Alam J., Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response pathway. Curr Pharm Des., 2003, 9, 2499–2511
Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol., 2013, 53, 401–426
Crespo I., Miguel B.S., Laliena A et al. Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J Pineal Res., 2010, 49(2), 193–200
Niture S.K., Jaiswal A.K. Nrf2-induced anti-apoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic Biol Med., 2012 Dec 27. doi:pii: S0891-5849(12)01864-3
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bekyarova, G., Tzaneva, M., Hristova, M. et al. Melatonin protection against burn-induced liver injury. A review. cent.eur.j.med 9, 148–158 (2014). https://doi.org/10.2478/s11536-013-0253-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11536-013-0253-7