Abstract
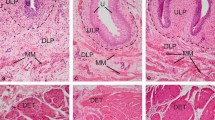
We compared the intensity of staining of interstitial cells (ICs) and neural tissue in the lower urinary tract of rabbits with diabetes with the intensity in normal subjects. Diabetes was induced by injecting alloxane (65mg/kg) in adult male rabbits. After 3 days, rabbits with a blood glucose level >300 mg/dL were considered to have diabetes. After 8 weeks, the rabbits were killed, and tissue specimens from the bladder, prostate and urethra were obtained. ICs were stained with anti-human CD117 (c-kit) rabbit polyclonal antibody, and neural tissue was stained with synaptophysin. The streptavidin-biotin method was used for immunohistochemical staining. The intensity of c-kit and synaptophysin staining were scored as negative (0), weak (+), moderate (++), and strong (+++). Staining intensity of ICs and neural tissue was assessed and compared in tissues obtained from rabbits with diabetes (n=8) and from control subjects (n=7). Although staining intensity of both ICs and neural tissue was found to be significantly decreased in the bladder tissue of rabbits with diabetes compared to that in the control group (p=0.0001 [ICs] and p=0.021 [neural tissue]), no significant differences in staining intensity of ICs and neural tissue in the urethra and in the prostate was found when rabbits with diabetes were compared to the control group. Diabetes may cause dysfunction of the lower urinary tract, particularly in the urinary bladder, as shown by the staining intensity of ICs and neural tissue.
Similar content being viewed by others
References
Aubert R.E., Geiss L.S., Ballard D.J., Cocanougher B., Herman W.H., Diabetes related hospitalization and hospital utilization., In: Diabetes in America, 2nd Edition: National Institutes of Health; 553–569,1995
Poladia D.P., Schanbacher B., Wallace L.J., Bauer J.A., Innervation and connexin isoform expression during diabetes-related bladder dysfunction: early structural vs. neuronal remodeling, Acta Diabetol., 2005,42(3),147–152
Kubota Y., Nakahara T., Mitani A., Maruko T., Sakamoto K., Ishii K., Augmentation of rat urinary bladder relaxation mediated by beta1-adrenoceptors in experimental diabetes, Eur J Pharmacol., 2003,467(1–3),191–195
Su X., Changolkar A., Chacko S., Moreland R.S., Diabetes decreases rabbit bladder smooth muscle contraction while increasing levels of myosin light chain phosphorylation, Am J Physiol Renal Physiol., 2004,287(4),690–699
Liu G., Lin Y.H., Yamada Y., Daneshgari F., External urethral sphincter activity in diabetic rats, Neurourol Urodyn., 2008,27(5),429–434
Yang Z., Dolber P.C., Fraser M.O., Diabetic urethropathy compounds the effects of diabetic cystopathy, J Urol., 2007,178(5),2213–2219
Torimoto K., Fraser M.O., Hirao Y., De Groat W.C., Chancellor M.B., Yoshimura N., Urethral dysfunction in diabetic rats, J Urol., 2004,171(5),1959–19564
van der AA F., Roskams T., Blyweert W., Ost D., Bogaert G., De Ridder D., Identification of kit positive cells in the human urinary tract, J Urol., 2004,171(6 Pt 1),2492–2496
Wiseman O.J., Fowler C.J., Landon D.N., The role of the human bladder lamina propria myofibroblast, BJU Int., 2003,91(1),89–93
Shafik A., El-Sibai O., Shafik A.A., Shafik I., Identification of interstitial cells of Cajal in human urinary bladder: concept of vesical pacemaker, Urology., 2004,64(4),809–813
Sergeant G.P., Hollywood M.A., McCloskey K.D., Thornbury K.D., McHale N.G.,Specialised pacemaking cells in the rabbit urethra, J Physiol., 2000,526,359–366
Seifert P., Benedic M., Effert P., Nerve fibers in tumors of the human urinary bladder, Virchows Arch., 2002,440(3),291–297
Felkl M., Leube R.E., Interaction assays in yeast and cultured cells confirm known and identify novel partners of the synaptic vesicle protein synaptophysin, Neuroscience., 2008,156(2),344–352
Wu J., Ohlsson M., Warner E.A., Loo K.K., Hoang T.X., Voskuhl R.R., et al., Glial reactions and degeneration of myelinated processes in spinal cord gray matter in chronic experimental autoimmune encephalomyelitis, Neuroscience., 2008,156(3),586–596
Hermann G.G., Andersen C.B., Transitional cell carcinoma Express vitamin D receptors, Scand J Urol Nephrol., 1996,31,161–166
Ristimaki A., Honkanen N., Jankala H., Sipponen P., Harkönen M., Expression of cyclooxygenase-2 in human gastric carcinoma, Cancer Res., 1997,57,1276–1280
Metzger R., Neugebauer A., Rolle U., Böhlig L., Till H., C-Kit receptor (CD117) in the porcine urinary tract, Pediatr Surg Int., 2008,24(1),67–76.
Kubota Y., Hashitani H., Shirasawa N., Kojima Y., Sasaki S., Mabuchi Y., Altered distribution of interstitial cells in the guinea pig bladder following bladder outlet obstruction, Neurourol Urodyn., 2008,27(4),:330–340
Højlund K., Mogensen M., Sahlin K., Beck-Nielsen H., Mitochondrial dysfunction in type 2 diabetes and obesity, Endocrinol Metab Clin North Am., 2008,37(3),713–731
Abdul-Ghani M.A., DeFronzo R.A., Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus, Curr Diab Rep., 2008,8(3),173–178
McCloskey KD., Characterization of outward currents in interstitial cells from the guinea pig bladder, J Urol., 2005,173,296–301
Canda AE., Editorial Comment on: Molecular Mechanisms Related to Parturition-Induced Stress Urinary Incontinence, Eur Urol., 2008,Mar 18, [Epub ahead of print]
Canda A.E., Cross R.L., Chapple C.R., Pharmacology of the lower urinary tract and management of overactive bladder, J Turkish-German Gynecol Assoc., 2006,7,146–158
Canda A.E., Cinar M.G., Turna B., Sahin M.O., Pharmacologic targets on the female urethra, Urol Int., 2008,80(4),341–354
Edwards F.R., Hirst G.D., Suzuki H., Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum, J Physiol., 1999,519,235–250
Sanders K.M., A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract, Gastroenterology., 1996,111,492–515
Collins C., Klausner A.P., Herrick B., Ko H.P., Miner A.S., Henderson S.C., Ratz P.H., Potential for control of detrusor smooth muscle spontaneous rhythmic contraction by cyclooxygenase products released by interstitial cells of Cajal, J Cell Mol Med., 2009, Feb 20, [Epub ahead of print]
Johnston L., Carson C., Lyons A.D., Davidson R.A., McCloskey K.D., Cholinergic-induced Ca2+ signaling in interstitial cells of Cajal from the guinea pig bladder, Am J Physiol Renal Physiol., 2008,294(3),645–655
Lyons A.D., Gardiner T.A., McCloskey K.D., Kit-positive interstitial cells in the rabbit urethra: structural relationships with nerves and smooth muscle, BJU Int. 2007,99(3),687–694
McCloskey K.D., Calcium currents in interstitial cells from the guinea-pig bladder, BJU Int., 2006,97(6),1338–1343
Nobe K., Yamazaki T., Tsumita N., Hashimoto T., Honda K., Glucose-dependent enhancement of diabetic bladder contraction is associated with a rho kinase-regulated protein kinase C pathway, J Pharmacol Exp Ther., 2009,328(3),940–950
Bradley W.E., Diagnosis of urinary bladder dysfunction in diabetes mellitus, Ann Intern Med 1980,92,323–326
Mitsui T., Kakizaki H., Kobayashi S., Morita H., Matsumura K., Koyanagi T., Vesicourethral function in diabetic patients: association of abnormal nerve conduction velocity with vesicourethral dysfunction, Neurourol Urodyn., 1999,18:639–645
Andersson K.E., Treatment-resistant detrusor overactivity—underlying pharmacology and potential mechanisms, Int J Clin Pract Suppl., 2006,(151),8–16
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Canda, A.E., Aktas, S., Turna, B. et al. Does diabetes affect the intensity of staining of interstitial cells and neuronal tissue in the bladder, prostate and urethra of rabbits?. cent.eur.j.med 5, 108–114 (2010). https://doi.org/10.2478/s11536-009-0064-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11536-009-0064-z