Abstract
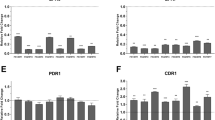
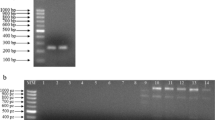
Candida albicans SAP4 gene encodes secretory aspartyl protease Sap4 which is involved in hyphae formation and virulence. Transcriptional factors Cph1 and Efg1 govern the expression of several C. albicans genes and contribute to morphogenesis. We investigated the expression of SAP4 in C. albicans clinical isolate and mutants lacking Efg1 or/ and Cph1 grown in human serum and during contact with Caco-2 cell line. mRNA was analyzed with the use of RT-PCR; relative quantification was normalized against an ACT1 in cells after 18-h growth either in serum or on monolayer as well as in their counterparts in YEPD medium. We assessed the role of Sap4, Efg1 and Cph1 in adhesion of C. albicans to epithelial cells. Additionally, adherence assay was performed with sap4/sap4. Adhesion was expressed as a percent of adherent cells to monolayer at 90 min vs. total cells added (100%). No differences were observed in adhesion of efg1/efg1 and sap4/sap4 compared with SC5314 (P≥0.05 statisitically insignificant). SAP4 expression indicated that it is not involved in adapting to the tested conditions. SAP4 expression can be strainspecific and is not solely controlled by the Efg1 pathway but also by the Cph1 pathway. Neither Efg1 nor Sap4 can influence adhesion.
Similar content being viewed by others
References
Sanchez A.A., Johnston D.A., Myers C., Edwards J.E., Mitchell A.P., Filler S.G., Relationship between Candida albicans virulence during experimental hematogenously disseminated infection and endothelial cell damage in vitro, Infect. Immun., 2004, 72, 598–601
Dalle F., Wächtler B., L’Ollivier C., Holland G., Bannert N., Wilson D., et al., Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes, Cell Microbiol., 2010, 12, 248–271
Langford M.L., Hargarten J.C., Patefield K.D., Marta E., Blankenship J.R., Fanning S., Nickerson K.W., Atkin A.L., Candida albicans Czf1 and Efg1 Coordinate the Response to Farnesol during Quorum Sensing, White-Opaque Thermal Dimorphism, and Cell Death. Eukaryot Cell., 2013 12, 1281–1292
Jackson B.E., Wilhelmus K.R., Hube B., The Role of Secreted Aspartyl Proteinases in Candida albicans Keratitis, Invest. Ophthal. Vis. Sci., 2007, 48, 3559–3565
Lo H.J., Köhler J.R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G.R., Nonfilamentous C. albicans Mutants Are Avirulent, Cell, 1997, 90, 939–949
Hube B., Fungal adaptation to the host environment, Curr. Opin. Microbiol., 2009, 12, 347–349
Martin R., Wächtler B., Schaller M., Wilson D., Hube B., Host-pathogen interaction and virulenceassociated genes during Candida albicans oral infections, Int. J. Med. Microbiol., 2011, 301, 417–422
Barnett J.A., A history of research on yeasts 12: medical yeasts part I, Candida albicans, Yeast, 2008, 25, 385–417
Staniszewska M., Bondaryk M., Siennicka K., Piłat J., Schaller M., Kurzątkowski W., Role of aspartic proteinases in Candida albicans virulence. Part II: expression of SAP1-10 aspartic proteinase during Candida albicans infectins in vivo, Post. Mikrobiol., 2012d, 51, 137–142
Staniszewska M., Bondaryk M., Swoboda-Kopeć E., Siennicka K., Sygitowicz G., Kurzątkowski W., Candida albicans morphogenesis revealed by scanning electron microscopy analysis, Braz. J. Microbiol., (in press), 2013, 3
Naglik J.R., Moyes D., Makwana J., Kanzaria P., Tsichlaki E., Weindl G., et al., Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis, Microbiology, 2008, 154, 3266–3280
Schweizer A., Rupp S., Taylor B.N., Röllinghoff M., Schröppel K., The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans, Mol. Microbiol., 2000, 38, 435–445
Wu H., Downs D., Ghosh K., Ghosh A.K., Staib P., Monod M., Tang J. Candida albicans secreted aspartic proteases 4–6 induce apoptosis of epithelial cells by a novel Trojan horse mechanism, FASEB J., 2013, 27, 2132–44
Correia A., Lermann U., Teixeira L., Cerca F., Botelho S., Gil da Costa R.M., Limited Role of Secreted Aspartyl albicans Virulence and Host Immune Proteinases Sap1 to Sap6 in Candida Response in Murine Hematogenously Disseminated Candidiasis. Infect. Immun., 2010, 78, 4839–4849
Pietrella D., Rachini A., Pandey N., Schild L., Netea M., Bistoni F., Hube B., Vecchiarelli A., The Inflammatory response induced by aspartic proteases of Candida albicans is independent of proteolytic activity. Infect Immun., 2010, 78, 4754–4762
White T.C., Agabian N., Candida albicans secreted aspartyl proteinases: isoenzyme pattern is determined by cell type, and levels are determined by environmental factors, J. Bacteriol., 1995, 177, 5215–5221
Staniszewska M., Bondaryk M., Kurek A., Orłowski J., Schaller M., Kurzątkowski W., In vito study of secreted aspartyl proteinases Sap1 to Sap3 and Sap4 to Sap6 expression in Candida albicans pleomorphic forms, Pol. J. Microbiol., 2012e, 61, 247–256
Fan Y., He H., Dong Y., Pan H., Hyphae-Specific Genes HGC1, ALS3, HWP1, and ECE1 and Relevant Signaling Pathways in Candida albicans, Mycopathologia, 2013
Moazenia M., Khoramizadeh M.R, Kordbacheh P., Sepehrizadeh Z., Zeraati H., Noorbakshsh F., Teimoori-Toolabi L., Rezaie S. RNA — Mediated gene silecing in Candida albicans: Inhibition of Hypae formation by use of RNAi technology, Mycopathologia, 2012, 174, 177–185
Wächtler B., Wilson D., Haedicke K., Dalle F., Hube B, From attachement to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells, PLOS ONE, 2011, 6, e17046
Chung S.C., Kim T.I., Ahn C.H., Shin J., Oh K.B., Candida albicans PHO81 is required for the inhibition of hyphal development by farnesoic acid, FEBS Letters, 2010, 584, 4639–4645
Han T-L., Cannon R.D., Villas-Bõas S.G., The metabolic basis of Candida albicans morphogenesis and quorum sensing, Fungal Genet. Biol., 2011, 48, 747–763
Staniszewska M., Bondaryk M., Siennicka K., Piłat J., Schaller M., Kurzątkowski W., Role of aspartic proteinases in Candida albicans virulence. Part I: Substrate specificity of aspartic proteinases and Candida albicans pathogenesis, Post. Mikrobiol., 2012c, 51, 127–135
Aoki W., Kitahara N., Miura N., Morisaka H., Yamamoto Y., Kuroda K., et al., Comprehensive characterization of secreted aspartic proteases encoded by a virulence gene family in Candida albicans, J. Biochem., 2011, 150, 431–438
Pinto M., Robine-Leon S., Appay M., Kedinger M., Triadou N., Dussaulx E., et al., Enterocyte like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture, Biol. Cell, 1983, 43: 323–330
Gillum A.M., Tsay E.Y., Kirsch D.R., Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations, Mol. Gen. Genet., 1984, 198, 179–182
Pfaller M.A., Bale M., Buschelman B., Lancaster M., Espinel-Ingroff A., Rex J.H., et al., Selection of Candidate Quality Control Isolates and Tentative Quality Control Ranges for In Vitro Susceptibility Testing of Yeast Isolates by National Committee for Clinical laboratory Standards Proposed Standard Methods, J. Clin. Microbiol., 1994, 32: 1650–1653
Staniszewska M., Search for Candida albicans virulence factors, PhD thesis, NIPH-NIH, Warsaw, Poland, 2009
Luo G., Mitchell T.G., Rapid identification of pathogenic fungi directly from cultures by using multiplex PCR. J. Clin. Microbiol., 2002, 40, 2860–2865
Ness F., Prouzet-Mauleon V., Vieillemard A., Lefebvre F., Noël T., Crouzet M., et al., The Candida albicans Rgd1 is a RhoGAP protein involved in the control of filamentous growth, Fungal Genet. Biol., 2010, 47, 1001–1011
Staniszewska M., Bondaryk M., Kurzątkowski W., Morphotypes of Candida albicans. Phase-contrast microscopy, Mikol. Lek., 2011, 18, 9–14
Staniszewska M., Bondaryk M., Siennicka K., Kurzątkowski W., Ultrastructure of Candida albicans Pleomorphic Forms: Phase-Contrast Microscopy, Scanning and Transmission Electron Microscopy, Pol. J. Microbiol., 2012a, 61, 129–135
Amberg D.C., Burke D.J., Strathern J.N., Yeast RNA isolations, Techniques and Protocols #6. In: Amberg D.C., Burke D.J., Strathern J.N., (Eds.) Methods in yeast genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2005, p. 127
Livak K.J., Schmittgen T.D., Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)), Method Methods, 2001, 25, 402–428
Hashash R., Younes S., Bahnan W., El Koussa J., Maalouf K., Dimassi H.I., et al., Characterisation of Pga1, a putative Candida albicans cell wall protein necessary for proper adhesion and biofilm formation, Mycoses, 2010, 54, 491–500
Correia A., Lermann U., Teixeira L., Cerca F., Botelho S., da Costa R.M., Sampaio P., Gärtner F., Morschhäuser J., Vilanova M., Pais C., Limited role of secreted aspartyl proteinases Sap1 to Sap6 in Candida albicans virulence and host immune response in murine hematogenously disseminated candidiasis. Infect. Immun., 2010, 78, 4839–49
Znaidi S., Nesseir A., Chauvel M., Rossignol T., d’Enfert C., A Comprehensive Functional Portrait of Two Heat Shock Factor-Type Transcriptional Regulators Involved in Candida albicans Morphogenesis and Virulence. PLoS Pathog., 2013, 9: e1003519
Kumamoto C.A., Vinces M.D., Contribution of hyphae and hypha-co-regulated genes to Candida albicans virulence, Cell. Microbiol., 2005, 7: 1546–1554
Jayatilake J.A.M.S. and Samaranayake L.P., Experimental superficial candidiasis on tissue models. Mycoses, 2010, 53, 285–295
Lermann U., Morschhauser J., Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans, Microbiology, 2008, 154, 3281–95
Moyes D.L., Murciano C., Runlall M., Kohli A., Islam A., Naglik J.R., Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med Microbiol Immunol, 2012, 201, 93–101
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Staniszewska, M., Bondaryk, M., Malewski, T. et al. The expression of the Candida albicans gene SAP4 during hyphal formation in human serum and in adhesion to monolayer cell culture of colorectal carcinoma Caco-2 (ATCC). cent.eur.j.biol. 9, 796–810 (2014). https://doi.org/10.2478/s11535-014-0311-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11535-014-0311-4