Abstract
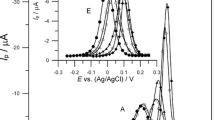
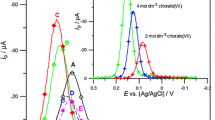
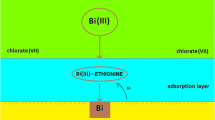
The results of the kinetic measurements of Bi(III) electroreduction on a mercury electrode in 1–8 mol dm−3 chlorate (VII) solutions and in the presence of cystine demonstrate a dependence of the process on the temperature. The applied electrochemical techniques (DC polarography, cyclic and SWV voltammetry) allowed for the determination of the kinetic and thermodynamic parameters and their correlation with water activity. The catalytic activity of cystine was confirmed by the decrease in overall enthalpies of activation. The changes in the values of ΔH ≠ and ΔS 0 for Bi(III) electroreduction in the presence of cystine with the increase of chlorate (VII) concentration showed that the mechanism is different in solutions with low water activity as compared to those with high water activity. Probably it is connected with a different structure of the activated complexes (Bi-Hg(SR)2), mediating electron transfer.

Similar content being viewed by others
References
Y.S. Ho, A.E. Ofomaja, Biochem. Eng. J. 30, 117 (2006)
A. Nosal-Wiercińska, G. Dalmata, Electroanalysis 14, 1275 (2002)
J.C. Lin, H.C. Shin, J. Electrochem. Soc. 134, 817 (1987)
V.V. Losev, A.I. Molodov, in: A.J. Bard (Ed.), Encyclopedia of Electrochemistry of the Elements (Marcel Dekker, New York, 1976)
J.W. Schultze, M.A. Habib, J. Appl. Electrochem. 9, 255 (1979)
A. Nosal-Wiercińska Cent. Eur. J. Chem. 8, 1 (2010)
J. Nieszporek, Monatsh. Chem. 141, 521 (2010)
J. Nieszporek, Croat. Chem. Acta 84, 103 (2011)
A. Nosal-Wiercińska, Electrochim. Acta, 55, 5917 (2010)
A. Nosal-Wiercińska, J. Electroanal. Chem. 654, 66 (2011)
A. Nosal-Wiercińska, J. Electroanal. Chem. 681, 103 (2012)
M. Wiśniewska, Powder Technology 198, 258 (2010)
M. Wiśniewska, Colloid and Polymer Science 289, 341 (2011)
V. Sharma, K. D. Gupta, Monatsh. Chem. 142, 481 (2011)
S. Marczak, P.K. Wrona, Z. Galus, J. Elactroanal. Chem. 471, 62 (1999)
G.G. López-Pérez, A. Andreu, D. González-Arjona, J.J. Calvente, M. Molero, J. Electroanal. Chem. 552, 247 (2003)
B. Marczewska, J. Włoszek, U. Kozłowska, Electroanalysis 11, 243 (1999)
W.R. Fawcett, J.S. Jaworski, J. Chem. Soc. Faraday Trans. I 78, 1971 (1982)
J. Nieszporek, J. Electroanal. Chem. 662, 407 (2011)
A. Nosal-Wiercińska, J. Electroanal. Chem. 662, 298 (2011)
A. Nosal-Wiercińska, Electrochim. Acta 92, 397 (2013)
M. Heyrovský, P. Mader, V. Veselá, M. Fedurco, J. Electroanal. Chem. 369, 53 (1994).
A. Nosal-Wiercińska, Cent. Eur. J. Chem. 10, 1290 (2012)
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nosal-Wiercińska, A. Influence of temperature on the reduction kinetics of Bi(III) ion in the presence of cystine in chlorate (VII) solutions of decreased water activity. cent.eur.j.chem. 12, 213–219 (2014). https://doi.org/10.2478/s11532-013-0376-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11532-013-0376-3