Abstract
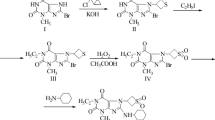
The synthesis of 3-deoxyoripavine (7) was realized as a novel and promising intermediate towards the synthesis of the important class of dopaminergic and/or serotonergic 10-deoxyaporphines and the special pharmacological tool µ opioid antagonist cyprodime. Generally, the preparation of these valuable biologically active compounds was achieved in remarkable yields.

Similar content being viewed by others
References
M.H. Hedberg, A.M. Johansson, G. Nordvall, A. Yliniemela, H.B. Li, A.R. Martin, S. Hjorth, L. Unelius, S. Sundell, H. Hacksell, J. Med. Chem. 38, 647 (1995)
Y.-G. Si, M.P. Gardner, F.I. Tarazi, R.J. Baldessarini, J.L. Neumeyer, J. Med. Chem. 51, 983 (2008)
Y.-G. Si, Y.-K. Choi, M.P. Gardner, F.I. Tarazi, R.J. Baldessarini, J.L. Neumeyer, Bioorg. Med. Chem. Lett. 19, 51 (2009)
Z. Liu, H. Zhang, N. Ye, J. Zhang, Q.Q. Wu, P. Sun, L. Li, X. Zhen, A. Zhang, J. Med. Chem. 53, 1319 (2010)
N. Ye, Q.Q. Wu, L. Zhu, L. Zheng, B. Gao, X. Zhen, A. Zhang, A. Bioorg. Med. Chem. 19, 1999 (2011)
A.W. Sromek, Y.-G. Si, T. Zhang, S.R. George, P. Seeman, J.L. Neumeyer, ACS Med. Chem. Lett. 2, 189 (2011)
V.J. Ram, J.L. Neumeyer, J. Het. Chem. 28, 1721 (1991)
J.G. Cannon, P. Mohan, J. Bojarski, J.P. Long, R.K. Bhatnagar, P.A. Leonard, J.R. Flynn, T.K. Chatterjee, J. Med. Chem. 31, 313 (1988)
J.G. Cannon, H. Jackson, J.P. Long, P. Leonard, R.K. Bhatnagar, J. Med. Chem. 32, 1959 (1989)
A.G. Millgate, B.J. Pogson, I.W. Wilson, T.M. Kutchan, M.H. Zenk, W.L. Gerlach, A.J. Fist, P.J. Larkin, Nature 431, 413 (2004)
S. Berényi, Cs. Csutorás, A. Sipos, Curr. Med. Chem. 16, 3215 (2009)
B.-S. Huang, on behalf of Penick Corp. US2008/0125592, 2008. CAN 148:472243
J.A.C. Alves, J.V. Barkley, A.F. Brigas, R.A.W. Johnstone, J. Chem. Soc., Perkin Trans. 2, 669 (1997)
I.D. Entwistle, B.J. Hussey, R.A.W. Johnstone, Tetrahedron Lett. 21, 4747 (1980)
B.J. Hussey, R.A.W. Johnstone, J.D. Entwistle, Tetrahedron 38, 3775 (1982)
D. Bethell, R.A.W. Johnstone, P.J. Price, J. Chem. Soc., Chem. Commun. 303 (1985)
R.A.W. Johnstone, W.N. McLean, Tetrahedron Lett. 29, 5553 (1988)
S. Cacchi, P.G. Ciattini, E. Morena, G. Ortar, Tetrahedron Lett. 27, 5541 (1986)
A. Mori, T. Mizusaki, T. Ikawa, T. Maegawa, Y. Monguchi, H. Sajiki, H. Chem. Eur. J. 13, 1432 (2007)
R. Bognár, Gy. Gaál, P. Kerekes, G. Horváth, M.T. Kovács, Org. Prep. Proc. Int. 6, 305 (1974)
L. Small, B.F. Faris, J. Am. Chem. Soc. 56, 1930 (1934)
L. Small, J. Org. Chem. 12, 359 (1947)
S. Berényi, S. Hosztafi, S. Makleit, I. Molnar, Acta Chim. Hung. 113, 51 (1983)
S.Y. Yeh, H.A. Krebs, C.W. Gorodetzky, J. Pharma. Sci. 68, 133 (1979)
Y. Gao, R.J. Baldessarini, N.S. Kula, J.L. Neumeyer, J. Med. Chem. 33, 1800 (1990)
S. Berényi, M. Czirják, S. Makleit, J. Chem. Soc. Perkin Trans. I. 2137 (1993)
S. Berényi, Cs. Csutorás, S. Makleit, F. Auth, I. Laszlovszky, B. Kiss, E. Kárpáti, M. Lőw, Med. Chem. Res. 7, 509 (1997)
M. Tóth, S. Berényi, Cs. Csutorás, N.S. Kula, K. Zhang, R.J. Baldessarini, J.L. Neumeyer, Bioorg. Med. Chem. 14, 1918 (2006)
A. Sipos, Cs. Csutorás, S. Berényi, A. Uustare, A. Rinken, A. Bioorg. Med. Chem. 16, 4563 (2008)
A. Sipos, S. Berényi, S. Synlett 11, 1703 (2008)
A. Sipos, L. Girán, H. Mittendorfer, H. Schmidhammer, S. Berényi, Tetrahedron 64, 1023 (2008)
V.J. Ram, J.L. Neumeyer, J. Het. Chem. 28, 1721 (1991)
Z. Liu, X. Chen, L. Yu, X. Zhen, A. Zhang, Bioorg. Med. Chem. 16, 6675 (2008)
J.L. Neumeyer, Y.-G. Si, A.W. Sromek on behalf of The McLean Hospital Corporation. WO 2011/130530 A1; 20/10/2011. CAN 155:563140
A. Udvardy, Zs. Gyulai, A. Sipos, J. Mol. Struct. 1002, 37 (2011) and References therein
H. Schmidhammer, W.P. Burkhard, L. Eggstein-Aeppli, C.F.C. Smith, J. Med. Chem. 32, 418, (1989)
F. Ötvös, G. Tóth, H. Schmidhammer, Helv. Chim. Acta 75, 1718 (1992)
H. Schmidhammer, A.E. Jacobson, L. Atwell, A. Brossi, Helv. Chem. Acta. 64, 2540 (1981)
R. Krassing, H. Schmidhammer, Heterocylcles 38, 877 (1994)
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the memory of Prof. Sándor Makleit who passed away on 27.09.2012
Electronic supplementary material
About this article
Cite this article
Sipos, A., Udvardy, A., Bényei, A.C. et al. The first synthesis of 3-deoxyoripavine and its utilization in the preparation of 10-deoxyaporphines and cyprodime. cent.eur.j.chem. 11, 1278–1285 (2013). https://doi.org/10.2478/s11532-013-0256-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11532-013-0256-x