Abstract
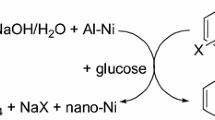
This article describes the simple hydrodehalogenation of halogenated anilines and their derivatives by the action of Raney aluminium-nickel alloy in aqueous alkaline solution at room temperature. The reaction course was monitored by means of 1H nuclear magnetic resonance (NMR) spectroscopy and GC-MS spectra.

The effect of Al and Ni and the nature and quantity of the base for effective hydrodehalogenation were studied.
The possibility of lowering Al content more than 500 times and Ni content more than 10 times in the filtered mother liquor by a dehalogenation procedure was tested using precipitation.
The reduction method described was satisfactorily proved for dehalogenation of polyhalogenated anilines in the multiphase dimethoxymethane/aqueous NaOH/Al-Ni reaction mixture. Dehalogenation under multi-phase conditions was demonstrated for the preparation of ortho-alkylated anilines from simply available 2-substituted-4-chloroanilines.
Similar content being viewed by others
References
F. Effenberger, Angew. Chem., Int. Ed. 41, 1699 (2002)
V.V. Grushin, Acc. Chem. Res. 26, 279 (1993)
H.Y. Choi, D.Y. Chi, J. Am. Chem. Soc. 123, 9202 (2001)
M.H. Block, S. Boyer, W. Brailsford, D.R. Brittain, D. Carroll, S. Chapman, D.S Clarke, C.S. Donald, K.M. Foote, L. Godfrey, A. Ladner, P.R. Marsham, D.J. Masters, C.D. Mee, M.R. O’Donovan, J.E. Pease, A.G. Pickup, J.W. Roberts, A. Rayner, P. Schofield, A. Suleman, A.V. Turnbull, J. Med. Chem. 45, 3509 (2002)
The Merck Index, An Encyclopedia of Chemicals, Drugs and Biologicals (Merck&Co., Inc., NI-159, New York, 1996) 640, 1209, 1239
E.C. Cortés, R.S. Franco, O.G. Mellado, J. Heterocyclic Chem. 38, 663 (2001)
L.T. Kaspar, B. Fingerhut, L. Ackermann, Angew. Chem., Int. Ed. 44, 5972 (2005)
N. Seshu Babu, K. Mohan Reddy, P.S. Sai Prasad, I. Suryanarayana, N. Lingaiah, Tetrahedron Lett. 48, 7642 (2007)
A. Arienti, F. Bigi, R. Maggi, E. Marzi, P. Moggi, Tetrahedron 53, 3795 (1997)
X.-T. Huang, Z.-Y. Long, Q.-Y. Chen, J. Fluorine Chem. 111, 107 (2001)
S.H. Yalkowsky, Y. He, Handbook of Aqueous Solubility Data (CRC Press, Boca Raton, Florida, 2003) 244
C. Tixier, M. Sancelme, F. Bonnemoy, A. Cuer, N. Truffaut, H. Veschambre, Environ. Toxicol. Chem. 20, 1381 (2001)
F. Massicot, R. Schneider, Y. Fort, S. Illy-Cherrey, O. Tillement, Tetrahedron 56, 4765 (2000)
F. Alonso, I.P. Beletskaya, M. Yus, Chem. Rev. 102, 4009 (2002)
G.-B. Liu, H.-Y. Zhao, B. Yang, T. Thiemann, Green Chem. Lett. Rev. 3, 1 (2010)
V. Dichiarante, M. Fagnoni, A. Albini, Green Chem. 11, 942 (2009)
X. Xu, H. Zhou, M. Zhou, Chemosphere 62(5), 847 (2006)
G. Lunn, E.B. Sansone, AIHA Journal 52, 252 (1991)
L.K. Keefer, G. Lunn, Chem. Rev. 89, 459 (1989)
G.-B. Liu, L. Dai, X. Gao, M.-K. Li, T. Thiemann, Green Chem. 8, 781 (2006)
G.-B. Liu, L. Dai, X. Gao, M.K. Li, T. Thiemann, Tetrahedron 65(12), 2497 (2009)
T. Weidlich, A. Krejčová, L. Prokeš, Monats. Chem. 141, 1015 (2010)
A.H.M. Veeken, W.H. Rulkens, Water Sci. Technol. 47(10), 9 (2003)
T.S. Roetting, J. Cama, C. Ayora, J.L. Cortina, J. De Pablo, Environ. Sci. Technol. 40, 6438 (2006)
J.Y. Lee, S.V. Rao, B.N. Kumar, D.J. Kang, B.R. Reddy, J. Hazard. Mater. 176, 1122 (2010)
A. Agueera, E. Almansa, A. Tejedor, A.R. Fenrandez-Alba, S. Malato, M.I. Maldonado, Environ. Sci. Technol. 34, 1563 (2000)
D. Quo, H. Huang, H. Jiang, H. Liu, J. Xu, Org. Lett. 10, 4513 (2008)
G. Manolikates, M.A. Schade, A. Metzger, P. Knochel, C. Munoz Hernandez, J. Org. Chem. 73, 8422 (2008)
M. Tordeux, B. Langlois, C.J. Wakselman, Chem. Soc., Perkin Trans. 1,8, 2293 (1990)
C.R. Gannelin, D.J. Triggle, Dictionary of pharmacological agents 1–2 (Chapman and Hall, London, 1996) 663
G.-B. Liu, H.-Y. Zhao, B. Yang, T. Thiemann, Green Chem. Lett. Rev. 3, 1 (2010)
B.H. Lipschutz, S. Tasler, Adv. Synth. Catal. 343, 327 (2001)
T. Janiak, J. Blazejowski, Chemosphere 48,1097 (2002)
C.J.H. Miermans, L.E. van der Velde, P.C.M. Frintrop, Chemosphere 40, 39 (2000)
W. Tsuzuki, A. Ue, A. Nagao, Biosci. Biotechnol. Biochem. 67, 1660 (2003)
H.E. Seifried, R.M. Seifried, J.J. Clarke, T.B. Junghans, R.H.C. San, Chem. Res. Toxicol. 19, 627 (2006)
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Weidlich, T., Prokeš, L. Facile dehalogenation of halogenated anilines and their derivatives using Al-Ni alloy in alkaline aqueous solution. cent.eur.j.chem. 9, 590–597 (2011). https://doi.org/10.2478/s11532-011-0033-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11532-011-0033-7