Abstract
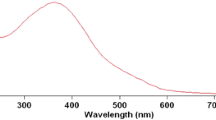
Kinetic measurements were performed for the alkaline hydrolysis of brilliant green — a triphenylmethane dye used as a model compound for probing micellar rate effects. This reaction was studied both in the presence of tetradecyltrimethylammonium bromide (TTAB) and tetradecyltriphenylphosphonium bromide (TTPPBr) and also in binary mixtures of these surfactants at different mole fractions of each. All rate surfactant profiles were analyzed using the pseudo-phase model in order to obtain the regression parameters, including binding constants and rate constants in the micellar pseudo-phase. The reaction was catalyzed by both surfactants. The catalytic factor increases from about 10 for pure TTPPBr to about 38 for pure TTAB. Binding of BG to micellar surface is greater in pure TTAB than in pure TTPPBr but significantly reduced in the surfactant mixtures than in pure components. Reduction of the binding constant becomes more significant as the mole fraction of TTAB is increased in the mixture. The kinetic data have been analyzed in terms of models of Piszkiewicz and Raghavan-Srinivasan which are in good agreement.

Similar content being viewed by others
References
K. Ogino, M. Abe (Eds.), Mixed Surfactant Systems (Dekker, New York, 1993)
M. El-Batanoney, T. Abdel-Moghny, M. Ramzi, J. Surf. Det. 2, 201 (1999)
M.J. Rosen, H. Wang, P. Shen, Y. Zhu, Langmuir 2, 3749 (2005)
A.E. Kharlov. G.P. Yampol’skaya, Moscow Univ. Chem. Bull. 62, 22 (2007)
F.T. Tadros, Applied Surfactants (Wiley-VCH Verlag GmbH and Co. KGaA, Weinheim, 2005)
T.J. Hall-Manning, G.H. Holland, G. Rennie, P. Revell, J. Hines, M.D. Barret, D.A. Basketter, Food Chem. Toxic. 36, 233 (1998)
Y. Yu, Z. Jin, A.E. Bayly, Chin. J. Chem. Eng. 16, 517 (2008)
T. Satsuki, Y. Nagoh, H. Yoshimura, J. Jap. Oil Chemist Soc. 48, 109 (1999)
J.L. Parra, J.J. Garcia-Dominuguez, A. de la Maza, J.S. Leal, J. Soc. Dyers Colourists 102, 227 (2008)
R.J. Goetz, M. El-Aasser, Langmuir 17, 993 (1990)
H. Hoffmann, G. Poessnecker, Langmuir 10, 381 (1994)
E. Marques, K. Khan, M. de-Miguel, B. Lindman, J. Phys. Chem. 97, 4729 (1993)
T. P. Goloub, R.J. Pugh, B.V. Zhmud, J. Colloid Interface Sci. 229, 72 (2000)
M. Bergstrom, Langmuir 17, 993 (2001)
M. Munoz, M. Rodriguez, M. D. Graciani, M.L. Moya, Int. J. Chem. Kinet. 34, 445 (2002)
M.N. Khan, E. Ismail, M.R. Yussof, J. Phys. Org. Chem. 14, 669 (2001)
G. Fernandez, A. Rodriguez, M. D. Graciani, M. Munoz, M.L. Moya, Int. J. Chem. Kinet. 35, 45 (2003)
H.M. Joshi, T.N. Nagar, Asian J. Chem. 14, 1763 (2002)
K.K. Ghosh, A. Pandey, J. Indian Chem. Soc. 76, 191 (1999)
R. Bacaloglu, A. Blasko, C.A. Bunton, G. Cerichelli, F. Ortega, J. Phys. Chem. 94, 5062 (1990)
R. Bacaloglu, C.A. Bunton, G. Cerichelli, F. Ortega, J. Phys. Chem. 94, 5068 (1990)
M.M. Mohareb, K.K. Ghosh, G. Orlova, R.M. Palepu, J. Phys. Org. Chem. 19, 281 (2006)
D.F. Duxbury, Chem. Rev. 93, 381 (1993)
B.M. Fox, G. Hallas, J.D. Hepworth, D. Mason, J. Chem. Tech. Biotech. 30, 317 (1980)
B.M. Fox, J.D. Hepworth, D. Mason, G. Hallas, J. Chem. Soc. (Perkin Trans. 2), 8, 987 (1982)
J.D. Hepworth, D.J. Lythgoe, D. Mason, G. Hallas, Dyes Pigments 15, 31 (1991)
O. Owoyomi, J. Ige, O. Soriyan, G. Ogunlusi, S.E. Olaseni, O. Olanrewaju, Acta Chim. Slov. 54, 370 (2007)
D.J. Jobe, V.C. Reinsborough, Aust. J. Chem. 37, 1593 (1984)
D. Piszkiewicz, J. Amer. Chem. Soc. 99, 1550 (1977)
A.V. Hill, J. Physiol. (London) 40, 4 (1910)
E. Pandey, S.K. Uphaday, Colloids Surf. A: Physicochem. Eng. Aspects 269, 7 (2005)
C.E. Drennan, R.J. Hughes, V.C. Reinsborough, O.O. Soriyan, Can. J. Chem. 76, 152 (1998)
P.S. Raghavan, V.S. Srinavasan, Proc. Indian Acad. Sci. (Chem. Sci.) 98, 199 (1987)
L.S. Romsted, In K.L. Mittal (Ed.), Micellization, Solubilization and Microemulsions (Plenum Press, New York, 1977) 2, 509
R.L. Reeves, J. Amer. Chem. Soc. 97, 6019 (1975)
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Olanrewaju, O., Ige, J. & Omopariola, S.O. Alkaline hydrolysis of brilliant green in mixed cationic surfactant systems. cent.eur.j.chem. 9, 106–111 (2011). https://doi.org/10.2478/s11532-010-0120-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11532-010-0120-1