Abstract
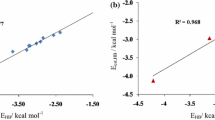
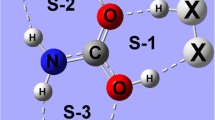
Ab initio calculations, including natural charge population and natural resonance theory analyses, have been carried out to study the two-way effects between hydrogen bonds (H-bonds) and the intramolecular resonance effect by using the H-bonded complexes of ring compounds containing the H2N-C=Y moiety (C=Y bond is contained in the six-membered or five-membered rings) with water as models. The amino groups in the four monomers of ring compounds (FAYs, Y represents the heavy atoms in the substituent groups, =CH, =N, =SiH, and =P, respectively) can all serve as H-bond donors (HD) and H-bond acceptors (HA) to form stable H-bonded complexes with water. The HD H-bond and resonance effect enhance each other (positive two-way effects) whereas the HA H-bond and resonance effect weaken each other (negative two-way effects). The resonance effect in FAY(1) (C=Y bond is contained in the six-membered rings) is weaker than that in formamide, and those in FAY(2) and FAY(3) (C=Y bonds are contained in the five-membered rings). The two-way effects between H-bond and resonance effect exist in the H-bonded complexes of ring compounds containing the H2N-C=Y moiety with water.

Similar content being viewed by others
References
A. Greenberg, C.M. Breneman, J.F. Liebman, The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science (Wiley, New York, 2000)
A. Greenberg, C.M. Breneman, J.F. Liebman, The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science (Wiley, New York, 2002)
L. Pauling, The Nature of the Chemical Bond, 3rd edition (Cornell University, Ithaca, New York, 1960)
E.D. Glendening, J.A. Hrabal, J. Am. Chem. Soc. 119, 12940 (1997)
T. Liu, H. Li, M.-B. Huang, Y. Duan, Z.-X. Wang, J. Phys. Chem. A 112, 5436 (2008)
S.F. Boys, F. Bernardi, Mol. Phys. 19, 553 (1970)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M. A. Robb, J.R. Cheeseman, J.A. Montgomery, Jr., T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A.G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B.G. Johnson, W. Chen, M.W. Wong, C. Gonzalez, J.A. Pople, GAUSSIAN 03, Revision B.04 (Gaussian, Inc., Pittsburgh, PA, 2003)
A.E. Reed, F. Weinhold, J. Chem. Phys. 78, 4066 (1983)
A.E. Reed, R.B. Weinstock, F. Weinhold, J. Chem. Phys. 83, 735 (1985)
F. Weinhold, J.E. Carpenter, In: R. Naaman, Z. Vager (Eds.), The Structure of Small Molecules and Ions (Plenum, New York, 1988) 227–236
E.D. Glendening, F. Weinhold, J. Comp. Chem. 19, 593 (1998)
E.D. Glendening, F. Weinhold, J. Comp. Chem. 19, 610 (1998)
E.D. Glendening, J.K. Badenhoop, F. Weinhold, J. Comp. Chem. 19, 628 (1998)
K.B. Wiberg, Tetrahedron 24, 1083 (1968)
E.D. Glendening, J.K. Badenhoop, A.E. Reed, J.E. Carpenter, B.J.A. Bohmann, C.M. Morales, F. Weinhold, NBO. 5.0 (Theoretical Chemistry Institute, University of Wisconsin, Madison, Wisconsin, 2001)
S. Scheiner, Hydrogen Bonding (Oxford University Press, New York, 1997)
G.R. Desiraju, T. Steiner, The Weak Hydrogen Bond (Oxford University Press, Oxford, 1999)
C.E. Dykstra, Acc. Chem. Res. 21, 355 (1988)
A.E. Reed, L.A. Curtiss, F. Weinhold, Chem. Rev. 88, 899 (1988)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
About this article
Cite this article
Liu, T., Liu, GD. & Yu, ZY. Ab initio study of hydrogen bond complexes of ring compounds containing the H2N-C=Y moiety with water. cent.eur.j.chem. 8, 1117–1126 (2010). https://doi.org/10.2478/s11532-010-0088-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11532-010-0088-x