Abstract
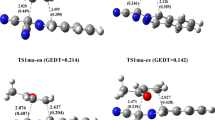
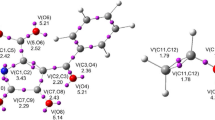
In this work, ab initio density functional theory (DFT) calculations have been performed on the 3,3-sigmatropic rearrangements of hexa-1,5-diene (Cope) and N-vinylprop-2-en-1-amine (3-aza-Cope) in the gas phase. The barrier heights and heats of reactions calculated at the B3LYP/6-311G** level of theory were in good agreement with experimental data. Transition states optimized with B3LYP/6-311G** theory were used for calculating the nucleus independent chemical shift (NICS) and, a natural bond orbital (NBO) analysis was also performed at the same level of theory. Our results indicate that the aromaticities of the transition states are controlled by the out-of-plane component and that the chair-like transition state of the Cope rearrangement exhibits the strongest aromatic character. Analysis of donor-acceptor (bonding and anti-bonding) interactions of σ3–4 → π*1–2 suggests that the TS structure in the hexa-1,5-diene reaction (the Cope rearrangement) has more aromatic character than the N-vinylprop-2-en-1-amine reaction (the 3-aza-Cope rearrangement). The NBO results show that in the hexa-1,5-diene and N-vinylprop-2-en-1-amine rearrangements, activation energies are controlled by σ3–4 → π*1–2 and σ3–4 → π*1–2 resonance energies.

Similar content being viewed by others
References
A.C. Cope, E.M. Hardy, J. Am. Chem. Soc. 62, 441 (1940)
M. Zora, J. Mol. Struct. (THEOCHEM) 681, 113 (2004)
F.A. Carey, R.J. Sundberg, Advanced Organic Chemistry, 5th edition (Springer, New York, 2007)
S. Sakai, J. Mol. Struct. (THEOCHEM) 583, 181 (2002)
V.N. Staroverov, E.R. Davidson, J. Mol. Struct. (THEOCHEM) 573, 81 (2001)
A.M.M. Castro, Chem. Rev. 104, 2939 (2004)
P. Merino, T. Tejro, V. Mannucci, Tetrahedron Lett. 48, 3385 (2007)
R.K. Hill, N.W. Gilman, Tetrahedron. Lett. 8, 1421 (1967)
S. Jolidon, H-J. Hansen, Helv. Chim. Acta. 60, 978 (1977)
M.J. Frisch et al., Gaussian 03, Revision D.01 (Gaussian Inc., Wallingford CT, 2004)
H.B. Schlegel, C. Peng, P.Y. Ayala, M.J. Frisch, J. Comput. Chem. 17, 49 (1996)
S. Glasstone, K.J. Laidler, H. Eyring, The Theory of Rate Processes (McGraw-Hill, New York, 1941)
K.J. Laidler, Theories of Chemical Reaction Rates (McGraw-Hill, New York, 1941)
S.W. Benson, F.R. Cruickshank, D.M. Golden, G.R. Haugen, H.E. O’Neal, A.S. Rodgers, R. Shaw, R. Walsh, Chem. Rev. 69, 279 (1969)
W.v.E. Doering, V.G. Toscano, G.H. Beasley, Tetrahedron. 27, 5299 (1971)
M. Hiersemann, U. Nubbemeyer, The Claisen Rearrangement: Methods And Applications (WILEYVCH Verlag GmbH & Co. KGaA, Weinheim, 2007)
N. Agmon, R.D. Levine, Chem. Phys. Lett. 52, 197 (1977)
P. Cysewski, J. Mol. Struct. (THEOCHEM) 714, 29 (2005)
P.V.R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N.J.R. van Eikema Hommes, J. Am. Chem. Soc. 118, 6317 (1996)
P.V.R. Schleyer, H. Jiao, B. Goldfuss, P.K. Freeman, Angew. Chem. Int. Ed. Engl. 34, 3337 (1995)
S. Nigam, C. Majumder, S.K. Kulshreshtha, J. Chem. Sci. 118, 575 (2006)
J.K. Badenhoop, F. Weinhold, Int. J. Quantum Chem. 72, 269 (1999)
J.E. Carpenter, F. Weinhold, J. Mol. Struct. (THEOCHEM) 169, 41 (1988)
E. Zahedi, M. Aghaie, K. Zare, J. Mol. Struct. (THEOCHEM). 905, 101 (2009)
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zahedi, E., Ali-Asgari, S. & Keley, V. NBO and NICS analysis of the allylic rearrangements (the Cope and 3-aza-Cope rearrangements) of hexa-1,5-diene and N-vinylprop-2-en-1-amine: A DFT study. cent.eur.j.chem. 8, 1097–1104 (2010). https://doi.org/10.2478/s11532-010-0084-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11532-010-0084-1