Abstract
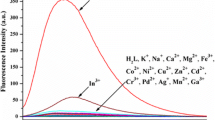
The ability of new chelate ligands, benzoxazol-5-yl-alanine derivatives substituted in position 2 by heteroaromatic substituent, to form complexes with selected metal ions in acetonitrile are studied by means of absorption and steady-state and time-resolved fluorescence spectroscopy. Among the ligands studied, only azaaromatic derivatives form stable complexes with transition metal ions in the ground state. Their absorption bands are bathochromically shifted enabling to use those ligands as ratiometric sensors. The fluorescence of each ligand is quenched by metal ions, however, in the presence of Cd(II) and Zn(II) ions a new red shifted emission band is observed.

Similar content being viewed by others
References
A.P. de Silva et al., Chem. Rev. 97, 1515 (1997)
B. Valuer, I. Leray, Coord. Chem. Rev. 205, 3 (2000)
J.F. Callan, A.P. de Silva, D.C. Magri, Tetrahedron 61, 8551 (2005)
K. Rurack, Spectrochim. Acta A 57, 2161 (2001)
C. Bargossi, M.C. Fiorini, M. Montalti, L. Prodi, N. Zaccheroni, Coord. Chem. Rev. 208, 17 (2000)
E.A. Medlycott, G.S. Hanan, Coord. Chem. Rev. 250, 1763 (2006)
F.N. Castellano, I.E. Pomestchenko, E. Shikhova, F. Hua, M.L. Muro, N. Rajapakse, Coord. Chem. Rev. 250, 1819 (2006)
G. Xue et al., Tetrahedron 58, 4809 (2002)
M.C. Kimber, I.B. Mahadevan, S.F. Lincoln, A.D. Ward, E.R.T. Tiekink, J. Org. Chem. 65, 8204 (2000)
C.J. Fahrni, T.V. O`Halloran, J. Am. Chem. Soc. 121, 11448 (1999)
G. Xue et al., Tetrahedron 57, 7623 (2001)
J.M. Castagnetto, J.W. Canary, Chem. Commun. 203 (1998)
Y. Mikata, M. Wakamatsu, S. Yano, Dalton Tran. 545 (2005)
D.-Y. Wu et al., Dalton Trans 29, 3528 (2006)
M.D. Shults, D.A. Pearce, B. Imperiali, J. Am. Chem. Soc. 125, 10591 (2003)
K. Hanaoka, K. Kikuchi, H. Kojima, Y. Urano, T. Nagano, J. Am. Chem. Soc. 126, 12470 (2004)
L. Xue, H.-H. Wang, X.-J. Wang, H. Jiang, Inorg. Chem. 47, 4310 (2008)
Z. Xu, X. Qian, J. Cui, R. Zhang, Tetrahedron 62, 10117 (2006)
K. Hanaoka, K. Kikuchi, H. Kojima, Y. Urano, T. Nagano, Angew. Chem. Int. Ed. 42, 2996 (2003)
V. Balzani, G. Bergamini, F. Marchioni, P. Ceroni, Coord. Chem. Rev. 250, 1254 (2006)
V. Amendola et al., Coord. Chem. Rev. 250, 273 (2006)
K. Tanaka, H. Kumagai, H. Aoki, M. Deguchi, S. Iwata, J. Org. Chem. 66, 7328 (2001)
J.-S. Youk, Y.H. Kim, E.-J. Kim, N.J. Youn, S.-K. Chang, Bull. Korean Chem. Soc. 25, 869 (2004)
M.G.B. Drew et al., New J. Chem. 28, 462 (2004)
K. Guzow et al., J. Photochem. Photobiol. A: Chem. 187, 87 (2007)
K. Guzow, M. Szabelski, J. Malicka, J. Karolczak, W. Wiczk, Tetrahedron 58, 2201 (2002)
SPECFIT/32 Global Analysis System for Windows, Spectrum Software Associates, Bio-Logic Science Instruments
K. Rurack, R. Radeglia, Eur. J. Inorg. Chem. 2271 (2000)
M.M. Henary, Y. Wu, C.J. Fahrni, Chem. Eur. J. 10, 3015 (2004)
K. Guzow, M. Milewska, D. Wróblewski, A. Giełdoń, W. Wiczk, Tetrahedron 60, 11889 (2004)
M. Milewska, A. Skwierawska, K. Guzow, D. Szmigiel, W. Wiczk, Inorg. Chem. Commun. 8, 947 (2005)
B. Valeur, In: J.R. Lakowicz (Ed.), Principles of Fluorescent Probe Design for Ion Recognition. In Topics in Fluorescence Spectroscopy, Probe Design and Chemical Sensing (Plenum Press, New York, 1994) vol. 4, 21–48
R. Snyder, A.C. Testa, J. Phys. Chem. 88, 5948 (1984)
H. Irving, R.J.P. Williams, Nature (London) 162, 746 (1948)
M.M. Henary, C.J. Fahrni, J. Phys. Chem. A 106, 5210 (2002)
M. Taki, J.L. Wolford, T.V. O’Halloran, J. Am. Chem. Soc. 126, 712 (2004)
C.J. Chang, J. Jaworski, E.M. Nolan, M. Sheng, S.J. Lippard, Proc. Natl. Acad. Sci. USA 101, 1129 (2004)
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Milewska, M., Guzow, K. & Wiczk, W. Fluorescent chemosensors for metal ions based on 3-(2-benzoxazol-5-yl)alanine skeleton. cent.eur.j.chem. 8, 674–686 (2010). https://doi.org/10.2478/s11532-010-0036-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11532-010-0036-9