Abstract
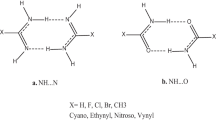
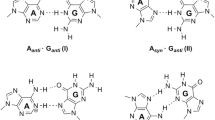
We have theoretically analyzed mimics of Watson-Crick AT and GC base pairs in which N-H···O hydrogen bonds are replaced by N-H···S, using the generalized gradient approximation (GGA) of density functional theory at BP86/TZ2P level. The general effect of the above substitutions is an elongation and a slight weakening of the hydrogen bonds that hold together the base pairs. However, the precise effects depend on how many, and in particular, on which hydrogen bonds AT and GC are substituted.. Another purpose of this work is to clarify the relative importance of electrostatic attraction versus orbital interaction in the hydrogen bonds involved in the mimics, using a quantitative bond energy decomposition scheme. At variance with widespread believe, the orbital interaction component in these hydrogen bonds is found to contribute more than 40% of the attractive interactions and is thus of the same order of magnitude as the electrostatic component, which provides the remaining attraction.

Similar content being viewed by others
References
P. Karran, Br. Med. Bull., 79–80, 153 (2006)
L. J. J. Derijks, L. P. L. Gilissen, P. M. Hooymans, D. W. Hommes, Aliment. Pharmacol. Ther., 24, 715 (2006)
E. B. Astwood, JAMA, 122 (1943) 78
L. Somerville et al., J. Biol. Chem., 278, 1005 (2003)
N. Spackova, E. Cubero, J. Sponer, M. Orozco, J. Amer. Chem. Soc., 126, 146421 (2004)
J. Sponer, J. Leszczynski, P. Hobza, J. Phys. Chem. A, 101, 9489 (1997)
S. Kawahara, T. Uchimaru, Eur. J. Org. Chem. 2577 (2003)
S. Kawahara, T. Uchimaru, K. Taira, M. Sekine, J. Phys. Chem. A, 106, 3207 (2002)
I. Dabkowska, P. Jurecka, P. Hobza, J. Chem. Phys. 122, art. 204322 (2005)
J. Sponer, P. Jurecka, P. Hobza, J. Am. Chem. Soc. 126, 10142 (2004)
P. Hobza, J. Sponer, Chem. Rev. 99, 3247 (1999)
J. Bertran, A. Oliva, L. Rodríguez-Santiago, M. Sodupe, J. Am. Chem. Soc. 120, 8159 (1998)
K. Brameld, S. Dasgupta, W. A. Goddard III, J. Phys. Chem. B 101, 4851 (1997)
J. Sponer, J. Leszczynski, P. Hobza, J. Phys. Chem., 100, 1965 (1996)
I. R. Gould, P. A. Kollman, J. Am. Chem. Soc., 116, 2493 (1994)
R. Santamaria, A. Vázquez, J. Comp. Chem., 15, 981 (1994)
J. Sponer, P. Hobza, J. Phys. Chem. A, 104, 4592 (2000)
P. Hobza, J. Sponer, E. Cubero, M. Orozco, F. J. Luque, J. Phys. Chem. B, 104, 6286 (2000)
J. Poater, X. Fradera, M. Solà, M. Duran, S. Simon, Chem. Phys. Lett., 369, 248 (2003)
C. Fonseca Guerra, F. M. Bickelhaupt, Angew. Chem., 111, 3120 (1999)
C. Fonseca Guerra, F. M. Bickelhaupt, Angew. Chem. Int. Ed., 38, 2942 (1999)
C. Fonseca Guerra, F. M. Bickelhaupt, J. G. Snijders, E. J. Baerends, J. Am. Chem. Soc., 122, 4117 (2000)
C. Fonseca Guerra, F. M. Bickelhaupt, J. G. Snijders, E. J. Baerends, Chem. Eur. J., 5, 3581 (1999)
C. Fonseca Guerra, E. J. Baerends and F. M Bickelhaupt, Crystal Growth & Design, 2, 239 (2002)
C. Fonseca Guerra, T. van der Wijst, F. M. Bickelhaupt, Chem. Eur. J., 12, 3032 (2006)
F. M. Bickelhaupt, E. J. Baerends, In: K. B. Lipkowitz and D. B. Boyd (Eds.) Rev. Comput. Chem. (Wiley-VCH: New York, 2000) 15, 1
L. Stryer, Biochemistry (W.H. Freeman and Company, New York, 1988) Chapter 1
D. Voet, J. G. Voet, Biochemistry (Wiley, New York, 1995) Chapter 2
G. A. Jeffrey, W. Saenger, Hydrogen Bonding in Biological Structures (Springer-Verlag, Berlin, 1991,) Chapter 2
G. A. Jeffrey, An Introduction to Hydrogen Bonding (Oxford University Press, New York, 1997) Chapter 2
G. R. Desiraju, T. Steiner, The Weak Hydrogen Bond (Oxford University Press, New York, 1999) Chapter 1
C. Fonseca Guerra, F. M. Bickelhaupt, Angew. Chem., 114, 2194 (2002)
C. Fonseca Guerra, F. M. Bickelhaupt, Angew. Chem. Int. Ed., 41, 2092 (2002)
C. Fonseca Guerra, F. M. Bickelhaupt, J. Chem. Phys., 119, 4262 (2003)
C. Fonseca Guerra, F. M. Bickelhaupt, E. J. Baerends, ChemPhysChem, 5, 481 (2004)
G. te Velde et al., J. Comput. Chem., 22, 931 (2001)
C. Fonseca Guerra, O. Visser, J. G. Snijders, G. te Velde, E. J. Baerends, In: E. Clementi and G. Corongiu (Eds.) Methods and Techniques for Computational Chemistry (STEF: Cagliari, 1995) 305
E. J. Baerends, D. E. Ellis, P. Ros, Chem. Phys., 2, 41 (1973)
E. J. Baerends, P. Ros, Chem. Phys., 8, 412 (1975)
E. J. Baerends, P. Ros, Int. J. Quantum. Chem. Symp., 12, 169 (1978)
C. Fonseca Guerra, J. G. Snijders, G. te Velde, E. J. Baerends, Theor. Chem. Acc., 99 391 (1998)
P. M. Boerrigter, G. te Velde, E. J. Baerends, Int. J. Quantum Chem., 33, 87 (1988)
G. te Velde, E. J. Baerends, J. Comp. Phys. 99, 84 (1992)
J. G. Snijders, E. J. Baerends, P. Vernooijs, At. Nucl. Data Tables, 26, 483 (1982)
J. Krijn, E. J. Baerends, Fit-Functions in the HFS-Method; Internal Report (in Dutch), Vrije Universiteit, Amsterdam, 1984
L. Versluis, T. Ziegler, J. Chem. Phys. 88, 322 (1988)
J. C. Slater, Quantum Theory of Molecules and Solids, Vol. 4, (McGraw-Hill, New York, 1974)
A. D. Becke, J. Chem. Phys., 84, 4524 (1986)
A. D. Becke, Phys. Rev. A, 38, 3098 (1988)
S. H. Vosko, L. Wilk, M. Nusair, Can. J. Phys., 58, 1200 (1980)
J. P. Perdew, Phys. Rev. B, 33, 8822 (1986) (Erratum: Phys. Rev. B, 34, 7406 (1986)
L. Fan, T. Ziegler, J. Chem. Phys., 94, 6057 (1991)
M. Swart, F.M. Bickelhaupt, J. Comput. Chem., (in press)
K. Morokuma, J. Chem. Phys., 55, 1236 (1971)
K. Kitaura, K. Morokuma, Int. J. Quantum. Chem., 10, 325 (1976)
T. Ziegler, A. Rauk, Inorg. Chem., 18, 1755 (1979)
T. Ziegler, A. Rauk, Inorg. Chem, 18, 1558 (1979)
T. Ziegler, A. Rauk, Theor. Chim. Acta, 46, 1 (1977)
F. M. Bickelhaupt, N. J. R. van Eikema Hommes, C. Fonseca Guerra, E. J. Baerends, Organometallics, 15, 2923 (1996)
C. Kittel, Introduction to Solid State Physics; Wiley: New York, (1986)
T. van der Wijst, C. Fonseca Guerra, M. Swart, F.M. Bickelhaupt, Chem. Phys. Lett., 426, 415 (2006)
For a proper comparison between theoretical A-T and G-C base-pairing enthalpies and mass spectrometric values, the latter must be corrected for the fact that they refer to a mixture of isomeric AT and GC complexes, respectively, which causes the experimental values to overestimate the Watson-Crick base-pairing enthalpies by about 1 kcal/mol (see ref [13], [22] and I. K. Yanson, A. B. Teplitsky, L. F. Sukhodup, Biopolymers, 18, 1149 (1979)
M. Swart, C. Fonseca Guerra, F. M. Bickelhaupt, J. Amer. Chem. Soc., 126, 16718 (2004)
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Guerra, C.F., Baerends, E.J. & Bickelhaupt, F.M. Watson-crick base pairs with thiocarbonyl groups: How sulfur changes the hydrogen bonds in DNA. cent.eur.j.chem. 6, 15–21 (2008). https://doi.org/10.2478/s11532-007-0068-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.2478/s11532-007-0068-y