Abstract
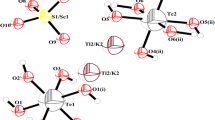
The crystal structure of tripotassium trisaccharinate dihydrate, K3(C7H4NO3S)3·2H2O, is triclic, space group\(P \bar 1, Z = 2\). It consists of three crystallographically independent potassium and saccharinato ions as well as two structurally different water molecules. Potassium coordination polyhedra are irregular, with K1 and K3 six-coordinated and the third one K2 seven-coordinated. The K−O distances range from 2.652(9) to 3.100(2) Å(mean: 2.790 Å) whereas the K−N distance is 3.025(3) Å. The water molecules W2 is disordered over three positions with occupancies of approximately 0.6, 0.2 and 0.2. The hydrogen atom (H1W1) of the ordered water molecule (O1W) is hydrogen bonded to the sulfonyl oxygen atom (O11) (R(O...O)=2.976(3) Å), whereas the other hydrogen atom (H2W1) is bifurcated to the carbonyl oxygen atom (O13) (R(O...O)=2.851(3) Å) and the disordered water molecules (O23W) (R(O...O)=3.067(12) Å). The carbonyl oxygens (O13, O23 and O33) and one of the disordered water molecules (O22W) are involved in C−H...O hydrogen bonds (R(C−H...O)=3.027(4)–3.304(9) Å). Structural characteristics of the studied compound are compared with the analogous trisodium trisaccharinate dihydrate and dipotassium sodium trisaccharinate monohydrate. Infrared and Raman spectra of the title compound have been analyzed in relation to the structure, and compared with the spectra of trisodium trisaccharinate dihydrate.
Similar content being viewed by others
References
J.M. Price, C.G. Biava, B.L. Oser, E.E. Vogin, J. Steinfeld and H.L. Ley: “Bladder Tumors in Rats Fed Cyclohexylamine or High Doses of a Mixture of Cyclamate and Saccharin”, Science, Vol. 167, (1970), pp. 1131–1132.
N. Suzuki, H. Suzuki: “Suppression of Saccharin-induced Mutagenicity by Interferon-α in Human RSa Cells”, Cancer Res., Vol. 55, (1995), pp. 4253–4256.
G. Jovanovski, B. Kamenar: “Two Ionic Saccharinates: (1a) Sodium Saccharinate 2/3 Hydrate, C7H4NO3SNa·2/3H2O; (1b) Magnesium Disaccharinate Heptahydrate, (C7H4NO3S)2 Mg·7H2O”, Cryst. Struct. Commun., Vol. 11, (1982), pp. 247–255.
K.M.A. Malik, S.Z. Haider and M.A. Hossain: “Dipotassium Sodium Trisaccharinate Monohydrate, K2Na(C7H4NO3S)3·H2O”, Acta Crystallogr. C., Vol. 40, (1984), pp. 1696–1698.
S.W. Ng, “Ammonium Saccharin”, Acta Crystallogr. C., Vol. 54, (1998), pp. 649–651.
S. Natarajan, G. Subramanian: “Infra-red Spectra of Sodium and Potassium Saccharinates”, Indian J. Phys. B., Vol. 60, (1986), pp. 470–473.
G. Jovanovski, O. Grupce and B. Soptrajanov: “The O−H and O−D Stretching Vibrations in the Hydrates of Sodium and Potassium Saccharinate: Spectra-structure Correlations”, J. Mol. Struct., Vol. 219, (1990), pp. 61–66.
S. Tanceva: “Infrared Spectra of Li, Ag, Sr, and Pb Saccharinates”, MSc Thesis (in Macedonian): “Sv. Kiril i Metodij” University, Faculty of Science, Institute of Chemistry, Skopje, 1994.
P. Naumov, G. Jovanovski: “Vibrational Study and Spectra-structure Correlations in Ammonium Saccharinate: Comparison with the Alkali Saccharinates”, Spectrochim. Acta A., Vol. 56, (2000), pp. 1305–1318.
A.C.T. North, D.C. Philips and F.S. Mathews: “A Semi-empirical Method of Absorption Correction”, Acta Crystallogr. A., Vol. 24, (1968), pp. 351–359.
International Tables for X-ray Crystallography, Vol. IV, Kynoch Press, Birmingham, 1974 pp. 99–101, 149–150. (Present Distributor Kluwer Academic Publishers, Dordrecht).
G.M. Sheldrick: “SHELXL97. Release 97-1. Program for the Refinement of Crystal Structures”, University of Göttingen, Germany, 1997.
L.J. Farrugia: “WinGX Suite for Small-molecule Single-crystal Crystallography”, J. Appl. Crystallogr., Vol. 32, (1999), pp. 837–838.
M.N. Burnett, C.K. Johnson: ORTEP-III, Report ORNL-6895., Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831-6285, USA, 1996.
A.I. Spek: PLUTON-93. Program for the Display and Analysis of Crystal and Molecular Structures, University of Utrecht, The Netherlands, 1993.
R.D. Shannon: “Revised Effective Ionic Radii and Systematic Studies of Interatomic Distance in Halides and Chalcogenides”, Acta Crystallogr. A., Vol. 32, (1976), pp. 751–767.
P. Naumov, G. Jovanovski, S. Abbrent and L.-E. Tergenius: “Thermal Behavior of the Saccharinates of K+, Na+, Rb+, Cs+ and NH +4 : Structural Inferences”, Thermochim. Acta, Vol. 359, (2000), pp. 123–130.
I.G. Binev, B. Stamboliyska and E. Velcheva: “The Infrared Spectra and Structure of o-sulfobenzimide (Saccharin) and of its Nitranion: An ab initio Force Field Treatment”, Spectrochim. Acta A., Vol. 52, (1996), pp. 1135–1143.
G. Jovanovski: “Metal Saccharinates and Their Complexes with N-donor Ligands”, Croat. Chem. Acta, Vol. 73, (2000), pp. 843–868.
G. Jovanovski, P. Naumov, O. Grupce and B. Kaitner: “Structural Study of Monoaquabis (pyridine) bis (saccharinato) copper(II), [Cu(H2O)(py)2(sac)2]”, Eur. J. Solid State Inorg. Chem., Vol. 35, (1998), pp. 231–242.
P. Naumov, O. Grupce and G. Jovanovski: “Experimental and Theoretical Raman Study of the Binuclear Copper(II) Imidazole Saccharinato Complex”, J. Raman Spectrosc., Vol. 31, (2000), pp. 475–479.
O. Grupce, G. Jovanovski and V. Mirceski: “Spectra-structure Correlations in 2,2′-bipyridine Mercury(II) Saccharinate: Comparison with Mercury(II) Saccharinate and Chloromercury(II) Saccharinate”, Spectrosc. Lett. Vol. 27, (1994), pp. 691–699.
I. Hase: “The Infrared and Raman Spectra of Phthalimide, N-d-Phthalimide and Potassium Phthalimide”, M. Mol. Struct., Vol. 48, (1978), pp. 33–42.
I. Hase: “The Infrared and Raman Spectra of Tetrachlorophthalic Anhydride, Tetrachlorophthalimide, N-d-Tetrachlorophthalimide and Potassium Tetrachlorophthalimide”, J. Mol. Struct., Vol. 52, (1979), pp. 163–173.
Author information
Authors and Affiliations
About this article
Cite this article
Jovanovski, G., Kaitner, B., Grupce, O. et al. Crystal structure, infrared and raman spectra of tripotassium trisaccha-rinate dihydrate, K3(C7H4NO3S)3·2H2O. cent.eur.j.chem. 2, 254–275 (2004). https://doi.org/10.2478/BF02476195
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.2478/BF02476195