Abstract
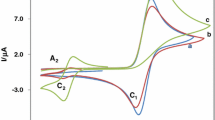
The kinetics of the oxidation of five catecholamines viz., dopamine (A), L-dopa (B), methyldopa (C), epinephrine (D) and norepinephrine (E) by sodium N-chloro-p-toluenesulfonamide or chloramine-T (CAT) in presence of HClO4 was studied at 30±0.1 °C. The five reactions followed identical kinetics with a first-order dependence on [CAT] o , fractional-order in [substrate] o , and inverse fractional-order in [H+]. Under comparable experimental conditions, the rate of oxidation of catecholamines increases in the order D>E>A>B>C. The variation of ionic strength of the medium and the addition of p-toluenesulfonamide or halide ions had no significant effect on the reaction rate. The rate increased with decreasing dielectric constant of the medium. The solvent isotope effect was studied using D2O. A Michaelis-Menten type mechanism has been suggested to explain the results. Equilibrium and decomposition constants for CAT-catecholamine complexes have been evaluated. CH3C6H4SO2NHCl of the oxidant has been postulated as the reactive oxidizing species and oxidation products were identified. An isokinetic relationship is observed with β=361 K, indicating that enthalpy factors control the reaction rate. The mechanism proposed and the derived rate law are consistent with the observed kinetics.
Similar content being viewed by others
References
G. Zubay: Biochemistry, 4th Ed., WCB, Boston, 1998.
J.G. Cory and T.M. Devlin: Text book of biochemistry with clinical correlations, 4th Ed., John Wiley and Sons, New York, 1997.
A.L. Lehninger, D.L. Nelson and M.M. Cox: Principles of biochemistry, 2nd Ed., CBS Publishers, New Delhi, 1993.
C.M. Lozano, T.P. Ruiz, V. Thomas and O. Val: “Determination of epinephrine, norepinephrine, dopamine and L-dopa in pharmaceutical by a photkinetic method”, Analyst, Vol. 116, (1991), p. 857.
E. Pelizzetti, E. Mentasti and E. Pramauro: “Kinetics and mechanism of oxidation pathways of some catecholamines with periodic acid”, J. Chem. Soc., Perkin Trans. 2, (1976), p. 1651.
B.S. Sherigara, E.V.S. Subrahmanyam, K. Ishwar Bhat and B.E. Kumaraswamy: “Oxidation of 3-(3,4-dihydroxy phenyl)-L-alanine (L-dopa) and 3-(3,4-dihydroxyphenyl)-2-methyl-L-alanine (methyldopa) by manganese (III) in pyrophosphate media: kinetic and mechanistic study”, Int. J. Chem. Kinet., Vol. 33(8), (2001), p. 449.
M.M. Campbell and G. Johnson: “Chloramine-T and related N-halogeno-N-metallo reagents”, Chem. Rev., Vol. 78, (1978), p. 65.
K.K. Banerji, B. Jayaram and D.S. Mahadevappa: “Mechanistic aspects of oxidation by N-metallo-N-haloarylsulfonamides”, J. Sci. Ind. Res., Vol. 46, (1987), p. 65.
Puttaswamy, D.S. Mahadavappa and K.S. Rangappa: “Oxidation of indigo carmine by N-haloarenesulfonamides: a kinetic study”, Bull. Chem. Soc. Jpn., Vol. 62, (1989), p. 3343.
U. Umeshkumar, K.C. Rajanna and P.K. Saiprakash: “A kinetic study of chloramine-T reaction with acetanilides in micellar media”, Pro. Nat. Acad. Sci. India, Vol. 65A, (1995), p. 279.
Puttaswamy, T.M. Anuradha, R. Ramachandrappa and N.M.M. Gowda: “Oxidation of isoniazide by N-haloarenesulfonamides in alkaline medium: A kinetic and mechanistic study”, Int. J. Chem. Kinet., Vol. 32(4), (2000), p. 221.
R.J.D. Saldanha, S. Ananda, B.M. Venkatesha and N.M.M. Gowda: “Oxidation of psychotropic drugs by chloaramine-T in acid medium: a kinetic study using spectrophotometry”, J. Mole. Str., Vol. 606, (2002), p. 147.
Puttaswamy, T.M. Anuradha and K.L. Mahadevappa: “Kinetic analysis of oxidation of dopamine by sodium N-chlorobenzenesulfonamide in perchloric acid medium: a mechanistic approach”, Indian J. Chem., Vol. 40A, (2001), p. 514.
Puttaswamy and R. Ramachandrappa: “Kinetics of dopamine oxidation by sodium N-bromo-p-toluenesulfonamide in acid medium: a mechanistic approach”, Oxid. Commun., Vol. 25 (1), (2002), p. 102.
Puttaswamy and Nirmala Vaz: “Kinetics and mechanism of ruthenium (III) and osmium (VIII) catalyzed oxidation of dopamine with bromamine-B in acid and alkaline media”, Stud. Surf.Sci. Cat., Vol. 133, (2001), p. 535.
J.C. Morris, J.R. Salazar and M.A. Winemann: “Equilibrium studies on chloro compounds: the ionization constant of N-chloro-p-toluenesulfonamide”, J. Am. Chem. Soc., Vol. 70, (1948), p. 2036.
G. Akerloff: “Dielectric constants of some organic solvents-water mixture at various temperatures”, J. Am. Chem. Soc., Vol. 54, (1932), p. 4125.
F. Feigl: Spot tests in organic analysis, 7th Ed., Elsevier, Amsterdam, 1966, pp. 332–335, 206.
A.I. Vogel: Text book of practical organic chemistry, 5th Ed., ELBS and Longman, London, 1966, p. 1257.
T.E. Young and B.W. Babbitt: “Electrochemical study of the oxidation of α-methyldopamine, α-methylnoradranaline and dopamine”, J. Org. Chem., Vol. 48, (1983) p. 562.
E. Bishop and V.J. Jennings: “Titrimetric analysis with chloramine-T: The status of chloramine-T as a titrimetric reagent”, Talanta, Vol. 1, (1958), p. 197.
F.F. Hardy and J.P. Johnston: “The interactions of N-bromo-N-sodiobenzesulfonamide (bromamine-B) with p-nitrophenoxide ion”, J. Chem. Soc., Perkin Trans.2, (1973), p. 742.
F.G. Soper: “The hydrolysis of the p-toluenesulfonchloroamides in water”, J. Chem. Soc. Trans., Vol. 125, (1924), p. 1899; (b) D.R. Pryde and F.G. Soper: “The interaction of anilides and hypochlorous acid”, J. Chem. Soc., (1931), p. 1510; (c) D.R. Pryde and F.G Soper: “The direct interchange of chlorine in the interaction of p-toluenesulfonamide and N-chloroactanilide”, J. Chem. Soc., (1931), p. 1514; (d) F.G. Soper and F.G. Smith: “The haloagenation of phenols”, J. Chem. Soc., (1926), p. 1582.
S.S. Narayanan and V.R.S. Rao: “Chlorine isotopic exchange reaction between chloramine-T and chloride ion”, Radio. Chim. Acta, Vol. 32, (1983), p. 211.
M. Subhashini, M. Subramanian and V.R.S. Rao: “Determination of the protonated constant of chloramine-B”, Talanta, Vol. 32, (1985), p. 1082.
J.E. House: Principles of chemical kinetics, Wm. C. Brown Publishers, Boston, 1997.
E.A. Moelwyn-Hughes: The kinetics of reaction in solutions, Clarendon Press, Oxford, 1947; Physical chemistry, 2nd Ed., Pergamon, New York, 1961.
S.W. Benson: The foundations of chemical kinetics, McGraw-Hill, New York, 1960.
A.A. Frost and R.G. Pearson: Kinetics and mechanism, 2nd Ed., Wiley, New York, 1961.
K.J. Laidler: Reaction kinetics, Pergamon, New York, 1963.
E.S. Amis: Solvent effects on reaction rates and mechanisms, Academic, New York, 1966.
S.G. Entelis and R.P. Tiger: Reaction kinetics in the liquid phase, Wiley, New York, 1966.
C.J. Collins and N.S. Bowman: Isotope effects in chemical reactions, Van Nostrand Reinhold, New York, 1970, p. 267.
K.B. Wiberg: Physical organic chemistry, Wiley, New York, 1964.
O. Exner: “Entropy-enthalpy compensation and anticompensation: solvation and ligand binding”, Chem. Commun., (2000), p. 1655 and references therein.
M. Anand Rao, B. Sethuram and Navaneeth Rao: “Oxidation studies: Ag(I)-catalysis in oxidation of amines of amines by Ce (IV) in nitric acid: A kinetic study”, J. Indian Chem. Soc., Vol. 59, (1982), p. 1040.
Puttaswamy and D.S. Mahadevappa: “Oxidation of substituted ethanols by sodium-N-bromobenzenesulfonamide: A kinetic study”, J. Phys. Org. Chem., Vol. 2, (1989), p. 660.
K.K. Senguptha, N. Bhattacharjee and B. Pal: “Kinetics and mechanism of the oxidation of neutralized α-hydroxy acids by tris(pyridine-2-carboxylato) manganese (III)”, Transition Metal. Chem., Vol. 24, (1999), p. 268.
K.S. Rangappa, K. Manjunathaswamy, M.P. Raghavendra and N.M.M. Gowda: “Kinetics and mechanism of oxidation of neutral α-aminoacids by sodium N-chloro-p-toluenesulfonamide in acid medium”, Int. J. Chem.Kinet., Vol. 34, (2002), p. 49.
Puttaswamy and Nirmala Vaz: “Kinetic analysis of oxidation of dipeptides by sodium N-bromobenzenesulfonamide in acid medium: a mechanistic approach”, Bull. Chem. Soc. Jpn., Vol. 76, (2003), p. 73.
Author information
Authors and Affiliations
About this article
Cite this article
Puttaswamy, Jagadeesh, R.V. & Vaz, N. Oxidation of some catecholamines by sodium N-chloro-p-toluenesulfonamide in acid medium: A kinetic and mechanistic approach. cent.eur.j.chem. 3, 326–346 (2005). https://doi.org/10.2478/BF02476000
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.2478/BF02476000