Abstract
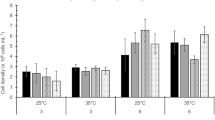
A 10−5 M (2.2 ppm) concentration of atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine) reduced the rate of photosynthesis, chlorophyll content, and cell numbers in unialgal cultures of Nitzschia sigma Grun. and Thalassiosira fluviatilis Hustedt isolated from a salt marsh habitat. Results with lower atrazine concentrations indicated an ability to maintain chlorophyll production and cell division with reduced photosynthesis. The effects of a 10−5 M concentration of atrazine in unialgal cultures were also evident in microecosystems and in the field at the same concentration, although atrazine effects were less severe in the field than in microecosystems or cultures. Cell number and productivity of the diatoms from microecosystems not treated with atrazine agreed well with field data and previously published data. Diatom species diversity was not affected by 10−5 M atrazine in microecosystems or in the field but the number of Cymatosira belgica Grun. was increased. Diatom assemblages in atrazine-treated vs non-treated microecosystems were very similar (SIMI>0.838). Results were less conclusive in the field but the trend was toward a lower level of similarity. Based on the least effect level of atrazine to diatoms, the maximum safe level for atrazine in the salt marsh is 10 ppb.
Similar content being viewed by others
Literature Cited
Admiraal, W.. 1977. Influence of light and temperature on the growth of estuarine benthic diatoms in culture. Mar. Biol. 39:1–9.
American Society for Testing and Materials Standards, Part 23. 1964. Tentative method of test for evaluating inhibitory toxicity of industrial waste waters, p. 517–525. Am. Soc. Test. Mater. Philadelphia, PA. 770 p.
Anonymous. 1977. Assessing Nonpoint Source Pollution: A Detailed Study of a Rural Watershed in the Coastal Plain of Maryland. Chesapeake Bay Center for Environmental Studies. Smithsonian Institution. July 1977. 59 p.
Couch, R. W., and D. E. Davis. 1966. Effect of atrazine, bromacil and diquat on C14O2-fixation in corn, cotton, and soybeans. Weeds 14:251–255.
Davis, D. E., C. G. P. Pillai, and B. Truelove. 1976. Effects of prometryn, diuron, fluometuron, and MSMA on Chlorella and two fungi. Weed Sci. 24:587–593.
Darley, W. M., E. L. Dunn, K. S. Holmes, and H. G. Larew, III. 1976. A 14C method for measuring epibenthic microalgal productivity in air. J. Exp. Mar. Biol. Ecol. 25:207–217.
—, C. T. Ohlman, and B. Wimpee. 1979. Utilization of dissolved organic carbon by natural populations of epibenthic salt marsh diatoms. J. Phycol. 15:1–5.
Eaton, J. W., and B. Moss. 1966. The estimation of numbers and pigment content in epipelic algal populations. Limnol. Oceanogr. 11:584–595.
Edwards, A. C., and D. E. Davis. 1974. Effects of herbicides on the Spartina salt marsh, p. 531–545. In R. J. Reimold and W. H. Queen (eds.), Ecology of Halophytes. Academic Press, New York.
Everest, J. W., and D. E. Davis. 1977. Microecosystems for herbicide research and other studies, p. 167–171. In B. Truelove (ed.). Research Methods in Weed Science, 2nd ed. South. Weed Sci. Soc. Auburn, AL.
Gallagher, J. L., and F. C. Daiber. 1974. Primary production of the edaphic algal communities in a Delaware salt marsh. Limnol. Oceanogr. 19:390–395.
Guillard, R. R. L. 1973. Division rates, p. 289–311. In. J. R. Stein (ed.). Handbook of Phycological Methods: Culture Methods and Growth Measurements. Cambridge Univ. Press.
—, And J. H. Ryther. 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 8:229–239.
Haines, E. B.. 1977. The origins of detritus in Georgia salt marsh estuaries. Oikos 29:254–260.
Hall, J. K., M. Pawlus, and E. R. Higgins. 1972. Losses of atrazine in run off water and soil sediment. J. Environ. Qual. 1:172–176.
Jorgensen, E. G.. 1964. Adaptation to different light intensities in the diatom Cyclotella meneghiniana Kutz. Physiol. Plant. 17:136–145.
McEnerney, J. T., and D. E. Davis 1979. Metabolic fate of atrazine in the Spartina alterniflora-detritus-Uca pugnax food chain. J. Environ. Qual. 8:335–338.
Patrick, R., and C. W. Reimer. 1966. The Diatoms of the United States. Vol. 1. Acad. Nat. Sci. Philadelphia. Monograph No. 13, 688 p.
Pillai, C. G. P., J. D. Weete, and D. E. Davis. 1977. Metabolism of atrazine by Spartina alterniflora. I. Chloroform-soluble metabolites. J. Agric. Food Chem. 25:852–855.
Plumley, F. G. 1978. Atrazine Effect on Salt Marsh Edaphic Algae. M.S. Thesis. Auburn University. 89 p.
Pomeroy, L. R. 1959. Algal productivity in salt marshes of Georgia. Limnol. Oceanogr. 4:386–397.
Pringsheim, E. 1946. Pure Cultures of Algae. Cambridge Univ. Press. 119 p.
Round, R. E.. 1971. Benthic marine diatoms. Oceanogr. Mar. Biol. Ann. Rev. 9:83–139.
Sullivan, M. J.. 1975. Diatom communities from a Delaware salt marsh. J. Phycol. 11:384–390.
—, And F. C. Daiber. 1975. Light, nitrogen, and phosphorus limitation of edaphic algae in a Delaware salt marsh. J. Exp. Mar. Biol. Ecol. 18:79–88.
Teal, J. M.. 1962. Energy flow in the salt marsh ecosystem of Georgia. Ecology 43:614–624.
Thompson, O. C., B. Truelove, and D. E. Davis. 1974. Effects of triazines on energy relations of mitochondria and chloroplasts. Weed Sci. 22:164–166.
Trainor, F. R.. 1978. Introductory Phycology. John Wiley & Sons, Inc. New York, 525 p.
Van Raalte, C. D., W. C. Stewart, I. Valiela, and E. J. Carpenter. 1974. A 14C technique for measuring algal productivity in salt marsh muds. Bot. Mar. 17:186–194.
—, I. Valiela, and J. M. Teal. 1976. Production of epibenthic salt marsh algae. Light and nutrient limitation. Limnol. Oceanogr. 21:862–872.
Williams, R. B. 1962. The Ecology of Diatom Populations in a Georgia Salt Marsh. Ph.D. Dissertation. Harvard University. 146 p.
WSSA Herb. Handbook Comm. 1974. Herbicide Handbook of the Weed Science Society of America, 3rd Ed. Weed Sci. Soc. Am., Champaigne, Ill., 430 p.
Yentsch, C. S., and D. W. Menzel. 1963. A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep-Sea Res. 10:221–231.
Author information
Authors and Affiliations
Additional information
Based in part on the Master of Science thesis of the senior author. The research was supported in part by funds made available by EPA under Grant No. R803-835-030.
Rights and permissions
About this article
Cite this article
Gerald Plumley, F., Davis, D.E. The effects of a photosynthesis inhibitor atrazine, on salt marsh edaphic algae, in culture, microecosystems, and in the field. Estuaries 3, 271–277 (1980). https://doi.org/10.2307/1352082
Issue Date:
DOI: https://doi.org/10.2307/1352082