Abstract
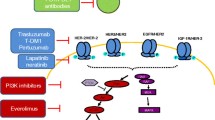
Human epidermal growth factor receptor (HER)-2 overexpression or amplification occurs in about 20% of all breast cancers and results in a worse prognosis. Nevertheless, anti-HER2 treatments have recently been developed, resulting in dramatic improvements in the clinical outcome of patients with HER2-positive breast cancer. Trastuzumab has shown efficacy in early and advanced breast cancer treatment and lapatinib is currently approved for the treatment of advanced disease. Other anti-HER2 agents are being investigated. Mechanisms of resistance to trastuzumab treatment include crosstalk with heterologous receptors and amplification of HER2 signalling; amplification of the phosphoinositide 3-kinase (PI3K)/AKT pathway; alteration in binding of trastuzumab to HER2; and loss of HER2 expression. Proposed mechanisms of resistance to lapatinib involve derepression and/or activation of compensatory survival pathways through increased PI3K/AKT or estrogen receptor (ER) signalling. Several strategies to overcome resistance to anti-HER2 treatment are in different phases of development and include treatment with pertuzumab, T-DM1 and mammalian target of rapamycin (mTOR) inhibitors.






Similar content being viewed by others
References
Sotiriou C, Pusztai L. Molecular origins of cancer gene-expression signatures in breast cancer. N Engl J Med 2009; 360: 790–800
Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009; 14: 320–68
Slamon DJ, Clark GM, Wong SG, et al. Human-breast cancer-correlation of relapse and survival with amplification of the her-2 neu oncogene. Science 1987; 235: 177–82
Slamon DJ, Godolphin W, Jones LA, et al. Studies of the Her-2/Neu proto-oncogene in human-breast and ovarian-cancer. Science 1989; 244: 707–12
Chia S, Norris B, Speers C, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol 2008; 26: 5697–704
Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 2010; 28: 92–8
Seshadri R, Firgaira FA, Horsfall DJ, et al. Clinical-significance of Her-2/Neu oncogene amplification in primary breast-cancer. J Clin Oncol 1993; 11: 1936–42
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–92
Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005; 23: 4265–74
Garnock-Jones KP, Keating GM, Scott LJ. Trastuzumab a review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. Drugs 2010; 70 (2): 215–39
Danese MD, Lalla D, Brammer M, et al. Estimating recurrences prevented from using trastuzumab in HER-2/neu-positive adjuvant breast cancer in the United States. Cancer 2010; 116: 5575–83
Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011; 29: 3366–73
Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol 2011; 12: 236–44
Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011; 365: 1273–83
Joensuu H, Bono P, Kataja V, et al. Update of the FINHER trial based on 5 years of follow-up [abstract no. S24]. Breast 2009; 18: S10
National Cancer Institute, France. Trastuzumab for 6 months or 1 year in treating women with nonmetastatic breast cancer that can be removed by surgery [Clinical-Trials.gov identifier NCT00381901]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 May 25]
Azienda Ospedaliera — Universitaria di Modena. Combination chemotherapy and trastuzumab in treating women with stage 1, stage II, or stage III HER2-positive breast cancer [ClinicalTrials.gov identifier NCT00629278]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 May 25]
Perez EA, Suman VJ, Davidson NE, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol 2011; 29: 4491–7
Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010; 375: 377–84
Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res 2007; 13: 228–33
Arpino G, Gutierrez C, Weiss H, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst 2007; 99: 694–705
Rimawi MF, Wiechmann LS, Wang YC, et al. Reduced dose and intermittent treatment with lapatinib and trastuzumab for potent blockade of the HER pathway in HER2/neu-overexpressing breast tumor xenografts. Clin Cancer Res 2011; 17: 1351–61
Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 2010; 28: 1124–30
Baselga J. BIEHeal: first results of the NeoALTTO trial (BIG 01-06/EGF 106903). A phase III, randomized, open label, neoadjuvant study of lapatinib, trastuzumab, and their combination plus paclitaxel in women with HER2-positive primary breast cancer. Cancer Res 2010; 70: 82
Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002; 2: 127–37
Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009; 69: 9330–6
Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 2010; 28: 1138–44
Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012 Jan; 13 (1): 25–32
Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012 Jan 12; 366 (2): 109–19
Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185(HER2) monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast. J Clin Oncol 1996; 14: 737–44
Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-over-expressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999; 17: 2639–48
Pegram MD, Konecny GE, O’Callaghan C, et al. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 2004; 96: 739–49
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Breast 2012; 1 [online]. Available from URL: http://www.nccn.com/files/cancer-guidelines/breast/index.html
Valero V, Forbes J, Pegram MD, et al. Multicenter phase iii randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 Study): two highly active therapeutic regimens. J Clin Oncol 2011; 29: 149–56
von Minckwitz G, Schweller K, Schmidt M, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer 2011; 47: 2273–81
von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol 2009; 27: 1999–2006
Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355: 2733–43
Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and bio-marker analyses. Breast Cancer Res Treat 2008; 112: 533–43
Hudis CA. Drug therapy: trastuzumab. Mechanism of action and use in clinical practice. N Engl J Med 2007; 357: 39–51
Baselga J, Albanell J, Molina MA, et al. Mechanism of action of trastuzumab and scientific update. Semin Oncol 2001; 28: 4–11
Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26: 1789–96
Gianni L. The ‘other’ signaling of trastuzumab: antibodies are immunocompetent drugs. J Clin Oncol 2008; 26: 1778–80
Collins DM, O’Donovan N, McGowan PM, et al. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann Oncol. Epub 2011 Nov 5
Molina MA, Codony-Servat J, Albanell J, et al. Trastuzumab (Herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Res 2001; 61: 4744–9
Klapper LN, Waterman H, Sela M, et al. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res 2000; 60: 3384–8
Levkowitz G, Oved S, Klapper LN, et al. c-Cbl is a suppressor of the Neu oncogene. J Biol Chem 2000; 275: 35532–9
Izumi Y, Xu L, di Tomaso E, et al. Tumor biology: Herceptin acts as an anti-angiogenic cocktail. Nature 2002; 416: 279–80
Pietras RJ, Pegram MD, Finn RS, et al. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 1998; 17: 2235–49
Lu YH, Zi XL, Zhao YH, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 2001; 93: 1852–7
Nahta R, Yuan LYH, Zhang B, et al. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 2005; 65: 11118–28
Jerome L, Alami N, Belanger S, et al. Recombinant human insulin-like growth factor binding protein 3 inhibits growth of human epidermal growth factor receptor-2-overexpressing breast tumors and potentiates Herceptin activity in vivo. Cancer Res 2006; 66: 7245–52
Browne BC, Crown J, Venkatesan N, et al. Inhibition of IGF1R activity enhances response to trastuzumab in HER-2-positive breast cancer cells. Ann Oncol 2011; 22: 68–73
Harris LN, You FL, Schnitt SJ, et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res 2007; 13: 1198–207
Kostler WJ, Hudelist G, Rabitsch W, et al. Insulin-like growth factor-1 receptor (IGF-1R) expression does not predict for resistance to trastuzumab-based treatment in patients with Her-2/neu overexpressing metastatic breast cancer. J Cancer Res Clin Oncol 2006; 132: 9–18
Lu YH, Zi XL, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int J Cancer 2004; 108: 334–41
Zhao YH, Liu H, Liu ZX, et al. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res 2011; 71: 4585–97
Shattuck DL, Miller JK, Carraway KL, et al. Met receptor contributes to trastuzumab resistance of Her2-over-expressing breast cancer cells. Cancer Res 2008; 68: 1471–7
Zhuang GL, Brantley-Sieders DM, Vaught D, et al. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res 2010; 70: 299–308
Liang K, Esteva FJ, Albarracin C, et al. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell 2010; 18: 423–35
Bieche I, Onody P, Tozlu S, et al. Prognostic value of ERBB family mRNA expression in breast carcinomas. Int J Cancer 2003; 106: 758–65
Holbro T, Beerli RR, Maurer F, et al. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A 2003; 100: 8933–8
Lee-Hoeflich ST, Crocker L, Yao E, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 2008; 68: 5878–87
Siegel PM, Ryan ED, Cardiff RD, et al. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J 1999; 18: 2149–64
Kim A, Liu BL, Ordonez-Ercan D, et al. Functional interaction between mouse erbB3 and wild-type rat c-neu in transgenic mouse mammary tumor cells. Breast Cancer Res 2005; 7: R708–18
Motoyama AB, Hynes NE, Lane HA. The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res 2002; 62: 3151–8
Moulder SL, Yakes FM, Muthuswamy SK, et al. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res 2001; 61: 8887–95
Cho HS, Mason K, Ramyar KX, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003; 421: 756–60
Ritter CA, Perez-Torres M, Rinehart C, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB Ligands and remain dependent on the ErbB receptor network. Clin Cancer Res 2007; 13: 4909–19
Adams CW, Allison DE, Flagella K, et al. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother 2006; 55: 717–27
Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004; 5: 317–28
Wang SE, Xian B, Guix M, et al. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol 2008; 28: 5605–20
Huang XP, Gao LZ, Wang SL, et al. Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-I receptor in breast cancer cells resistant to Herceptin. Cancer Res 2010; 70: 1204–14
Campbell IG, Russell SE, Choong DYH, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res 2004; 64: 7678–81
Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther 2004; 3: 772–5
Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 2007; 448: 439-U1
Bellacosa A, Defeo D, Godwin AK, et al. Molecular alterations of the Akt2 oncogene in ovarian and breast carcinomas. Int J Cancer 1995; 64: 280–5
Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997; 275: 1943–7
Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A 2007; 104: 7564–9
Gewinner C, Wang ZGC, Richardson A, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell 2009; 16: 115–25
Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 2005; 65: 2554–9
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. Integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 2008; 68: 6084–91
Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007; 12: 395–402
Nagata Y, Lan KH, Zhou XY, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004; 6: 117–27
Fujita T, Kawasaki K, Takabatake D, et al. PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer 2006 Jan 30; 94 (2): 247–52
Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res 2008; 68: 8022–30
Eichhorn PJA, Gili M, Scaltriti M, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res 2008; 68: 9221–30
Chakrabarty A, Rexer BN, Wang SE, et al. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene 2010; 29: 5193–203
Dalenc F, Campone M, Hupperets P, et al. Everolimus in combination with weekly paclitaxel and trastuzumab in patients (pts) with HER2-overexpressing metastatic breast cancer (MBC) with prior resistance to trastuzumab and taxanes: a multicenter phase II clinical trial [abstract no. 1013]. J Clin Oncol 2010; 28: 15 Suppl
Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol 2011; 29: 3126–32
Price-Schiavi SA, Jepson S, Li P, et al. Rat MUC4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for Herceptin resistance. Int J Cancer 2002; 99: 783–91
Nagy P, Friedlander E, Tanner M, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res 2005; 65: 473–82
Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 2007; 99: 628–38
Molina MA, Saez R, Ramsey EE, et al. NH2-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res 2002; 8: 347–53
Christianson TA, Doherty JK, Lin YJ, et al. NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res 1998; 58: 5123–9
Codony-Servat J, Albanell J, Lopez-Talavera JC, et al. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res 1999; 59: 1196–201
Anido J, Scaltriti M, Serra JJ, et al. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J 2006; 25: 3234–44
Saez R, Molina MA, Ramsey EE, et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res 2006; 12: 424–31
Pedersen K, Angelini PD, Laos S, et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol Cell Biol 2009; 29: 3319–31
Scaltriti M, Chandarlapaty S, Prudkin L, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res 2010; 16: 2688–95
Kwong KY, Hung MC. A novel splice variant of HER2 with increased transformation activity. Mol Carcinog 1998; 23: 62–8
Castiglioni F, Tagliabue E, Campiglio M, et al. Role of exon-16-deleted HER2 in breast carcinomas. Endocr Relat Cancer 2006; 13: 221–32
Mitra D, Brumlik MJ, Okamgba SU, et al. An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol Cancer Ther 2009; 8: 2152–62
Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res 2009; 15: 7381–812
Pectasides D, Gaglia A, Arapantoni-Dadioti P, et al. HER-2/neu status of primary breast cancer and corresponding metastatic sites in patients with advanced breast cancer treated with trastuzumab-based therapy. Anticancer Res 2006; 26: 647–53
Hurley J, Doliny P, Reis I, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer [published erratum appears in J Clin Oncol 2005 Jul 20; 24 (21): 3515]. J Clin Oncol 2006; 24 (12): 1831–8
Gomez HL, Doval DC, Chavez MA, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol 2008; 26: 2999–3005
Faber AC, Wong KK, Engelman JA. Differences underlying EGFR and HER2 oncogene addiction. Cell Cycle 2010; 9: 851–2
Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 2010; 28: 1301–7
Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A 2006; 103: 7795–800
Guo SQ, Sonenshein GE. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the Her-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol Cell Biol 2004; 24: 8681–90
Xia WL, Bacus S, Husain I, et al. Resistance to ErbB2 tyrosine kinase inhibitors in breast cancer is mediated by calcium-dependent activation of RelA. Mol Cancer Ther 2010; 9: 292–9
Liu L, Greger J, Shi H, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res 2009; 69: 6871–8
Hafizi S, Dam B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev 2006; 17: 295–304
Sergina NV, Rausch M, Wang DH, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007; 445: 437–41
Amin DN, Sergina N, Ahuja D, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med 2010 Jan 27; 2 (16): 16ra7
Press MF, Finn RS, Cameron D, et al. HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res 2008; 14: 7861–70
Freeman D, Ogbagabriel S, Rothe M, et al. Fully human anti-HER3 monoclonal antibodies (mAbs) have unique in vitro and in vivo functional and anti-tumor activities versus other HER family inhibitors. Proc Am Assoc Cancer Res 2008; 49
Phillips GDL, Li GM, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an anti-body-cytotoxic drug conjugate. Cancer Res 2008; 68: 9280–90
Junttila TT, Li GM, Parsons K, et al. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011; 128: 347–56
Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 2010; 28: 2698–704
Burris HA, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2) -positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011; 29: 398–405
Hurvitz S, Dirix L, Kocsis J, et al. Trastuzumab emtansine (T-DM1) vs trastuzumab plus docetaxel (H+T) in previously-untreated HER2-positive metastatic breast cancer (MBC): primary results of a randomized, multicenter, open-label phase II study (TDM4450 g/BO21976). Eur J Cancer 2011; 47: S330
Chandarlapaty S, Scaltriti M, Angelini P, et al. Inhibitors of HSP90 block p95-HER2 signaling in trastuzumabresistant tumors and suppress their growth. Oncogene 2010; 29: 325–34
Modi S, Stopeck A, Linden H, et al. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res 2011; 17: 5132–9
Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009; 9: 550–62
Acknowledgements
The authors thank Dr Roberta Sottile for her assistance in preparing the manuscript.
Dr Fabio Puglisi has received consulting fees or honorarium from Roche and GlaxoSmithKline. Dr Grazia Arpino has received consulting fees or honorarium from Roche and GlaxoSmithKline. All other authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puglisi, F., Minisini, A.M., De Angelis, C. et al. Overcoming Treatment Resistance in HER2-Positive Breast Cancer. Drugs 72, 1175–1193 (2012). https://doi.org/10.2165/11634000-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11634000-000000000-00000