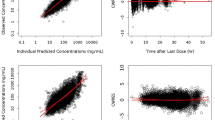
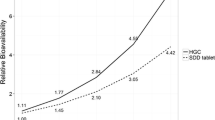
Abstract
Background and Objective
Danoprevir, a potent, selective inhibitor of the hepatitis C virus (HCV) NS3/4A protease, is metabolized by cytochrome P450 (CYP) Clinical studies in HCV patients have shown a potential need for a high danoprevir daily dose and/or dosing frequency. Ritonavir, an HIV-1 protease inhibitor (PI) and potent CYP3 A inhibitor, is used as a pharmacokinetic enhancer at subtherapeutic doses in combination with other HIV PIs. Coadministering danoprevir with ritonavir as a pharmacokinetic enhancer could allow reduced danoprevir doses and/or dosing frequency. Here we evaluate the impact of ritonavir on danoprevir pharmacokinetics.
Methods
The effects of low-dose ritonavir on danoprevir pharmacokinetics were simulated using Simcyp, a population-based simulator. Following results from this drug-drug interaction (DDI) model, a crossover study was performed in healthy volunteers to investigate the effects of acute and repeat dosing of low-dose ritonavir on danoprevir single-dose pharmacokinetics. Volunteers received a single oral dose of danoprevir 100 mg in a fixed sequence as follows: alone, and on the first day and the last day of 10-day dosing with ritonavir 100 mg every 12 hours.
Results
The initial DDI model predicted that following multiple dosing of ritonavir 100 mg every 12 hours for 10 days, the danoprevir area under the plasma concentration-time curve (AUC) from time zero to 24 hours and maximum plasma drug concentration (Cmax) would increase by about 3.9- and 3.2-fold, respectively. The clinical results at day 10 of ritonavir dosing showed that the plasma drug concentration at 12 hours postdose, AUC from time zero to infinity and Cmax of danoprevir increased by approximately 42-fold, 5.5-fold and 3.2-fold, respectively, compared with danoprevir alone. The DDI model was refined with the clinical data and sensitivity analyses were performed to better understand factors impacting the ritonavir-danoprevir interaction.
Conclusion
DDI model simulations predicted that danoprevir exposures could be successfully enhanced with ritonavir coadministration, and that a clinical study confirming this result was warranted. The clinical results demonstrate that low-dose ritonavir enhances the pharmacokinetic profile of low-dose danoprevir such that overall danoprevir exposures can be reduced while sustaining danoprevir trough concentrations.






Similar content being viewed by others
References
Seiwert SD, Andrews SW, Jiang Y, et al. Preclinical characteristics of the hepatitis C virus NS3/4A protease inhibitor ITMN-191 (R7227). Antimicrob Agents Chemother 2008 Dec; 52(12): 4432–41
Bradford WZ, Rubino C, Porter S, et al. A phase 1 study of the safety, toler-ability, and pharmacokinetics of single ascending oral doses of the NS3/4A protease inhibitor ITMN-191 in healthy subjects [abstract]. Hepatology 2008 Oct; 48 Suppl. 1: 1146A
Rubino C, Bradford WZ, Forrest A, et al. Pharmacokinetic-pharmacodynamic (PK-PD) relationships for ITMN-191 in a phase 1 multiple ascending dose trial in patients with genotype 1 chronic hepatitis C infection [abstract]. Hepatology 2008 Oct; 48 Suppl. 2: 1140A–1A
Forestier N, Larrey DG, Guyader D, et al. Treatment of chronic hepatitis C patients with the NS3/4A protease inhibitor danoprevir (ITMN-191/RG7227) leads to robust reductions in viral RNA: a phase 1b multiple ascending dose study. J Hepatol 2011 Jun; 54: 1130–6
Forestier N, Larrey D, Marcellin P, et al. Antiviral activity and safety of ITMN-191 in combination with peginterferon alfa-2a and ribavirin in patients with chronic C virus (HCV) [abstract]. J Hepatol 2009; 50 Suppl. 1: S35
Terrault N, Cooper C, Balart LA, et al. Phase II randomised, partially-blind, parallel-group study of oral danoprevir (RG7227) with PegIFN α-2a (PEGASYS®) plus ribavirin in treatment-naive genotype 1 patients with CHC: results of planned week 12 interim analysis of the ATLAS study [abstract]. Hepatology 2010 Oct; 52 Suppl. 4: 335A
Levi M, Frey N, Hsu JC, et al. High exposure to danoprevir (RG7227) increases the probability of ALT elevations in patients treated with danoprevir plus peginterferon alfa-2a (40KD) (PEGASYS®) plus ribavirin [abstract]. Hepatology 2010 Oct; 52 Suppl. 4: 1216A
Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother 2004 Jan; 53(1): 4–9
Moyle GJ, Back D. Principles and practice of HIV-protease inhibitor pharmacoenhancement. HIV Med 2001 Apr; 2(2): 105–13
Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother 2008 Jul; 42(7): 1048–59
Data on file, Roche, 2009
Grime KH, Bird J, Ferguson D, et al. Mechanism-based inhibition of cytochrome P450 enzymes: an evaluation of early decision making in vitro approaches and drug-drug interaction prediction methods. Eur J Pharm Sci 2009 Feb 15; 36(2–3): 175–91
Simcyp. A guide for IVIVE and PBPK modelling using the Simcyp population-based ADME simulator. Sheffield: Simcyp Limited, 2010: 394
Yang JS, Jamei M, Yeo KR, et al. Prediction of intestinal first-pass drug metabolism. Curr Drug Metab 2007 Oct; 8: 676–84
Gao H, Steyn SJ, Chang G, et al. Assessment of in silico models for fraction of unbound drug in human liver microsomes. Expert Opin Drug Metab Toxicol 2010 May; 6(5): 533–42
Turner DB, Yeo KR, Tucker GT, et al. Prediction of non-specific hepatic microsomal binding from readily available physicochemical properties [abstract no. A231]. Drug Metab Rev 2006; 38(1): 162
Cozza KL, Armstrong SC, Oesterheld JR. Concise guide to drug interaction principles for medical practice: cytochrome P450s, UGTs, P-glycoproteins. Arlington (VA): American Psychiatric Publishing, 2003 Jan
Almond L, Yeo KR, Howgate E, et al. Mechanistic prediction of HIV drug-drug interactions in virtual populations from in vitro enzyme kinetic data: ritonavir and saquinavir. International Workshop on Clinical Pharmacology of HIV Therapy, Budapest, Hungary, 16th–18th April 2007 [online]. Available from URL: http://www.simcyp.com/UploadedFiles/154/Lisa_Almond_2007_Budapest_SQVr_finalised.pdf [Accessed 2011 Nov 10]
Hyland R, Dickins M, Collins C, et al. Maraviroc: in vitro assessment of drug-drug interaction potential. Br J Clin Pharmacol 2008 Oct; 66: 498–507
Annaert P, Ye ZW, Stieger B, et al. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica 2010; 40: 163–76
Jackson A, Watson V, Back D, et al. Plasma and intracellular pharmacokinetics of darunavir/ritonavir once daily and raltegravir once and twice daily in HIV-infected individuals. J Acquir Immune Defic Syndr 2011 Dec 15; 58(5): 450–7
Wang YH. Confidence assessment of the Simcyp time-based approach and a static mathematical model in predicting clinical drug-drug interactions for me-chanism-based CYP3A inhibitors. Drug Metab Dispos 2010 Apr; 38: 1094–104
Gane EJ, Rouzier R, Stedman C, et al. Antiviral activity, safety, and pharmacokinetics of danoprevir/ritonavir plus PEG-IFN α-2a/RBV in hepatitis C patients. J Hepatol 2011 Nov; 55(5): 972–9
Gabrielsson J, Weiner D. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications. 4th ed. Stockholm: Swedish Pharmaceutical Press, 2007 Jul
Mathias AA, German P, Murray BP, et al. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin Pharmacol Ther 2010 Mar; 87(3): 322–9
Hill A, van der Lugt J, Sawyer W, et al. How much ritonavir is needed to boost protease inhibitors? Systematic review of 17 dose-ranging pharmacokinetic trials. AIDS 2009 Nov 13; 23(17): 2237–45
Hsu A, Granneman GR, Bertz RJ. Ritonavir: clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet 1998 Oct; 35: 275–91
Kenny JR, McGinnity DF, Grime K, et al. Utilization of in vitro cytochrome P450 inhibition data for projecting clinical drug-drug interactions. In: Gad SC, editor. Preclinical development handbook: ADME and biopharmaceutical properties. Hoboken (NJ): Wiley, 2008 Aug: 775–828
Poirier A, Cascais AC, Funk C, et al. Prediction of pharmacokinetic profile of valsartan in human based on in vitro uptake transport data. J Pharmacokinet Pharmacodyn 2009; 36: 585–611
Watanabe T, Kusuhara H, Sugiyama Y. Application of physiologically based pharmacokinetic modeling and clearance concept to drugs showing trans-porter-mediated distribution and clearance in humans. J Pharmacokinet Pharmacodyn 2010 Dec; 37(6): 575–90
Reesink HW, Zeuzem S, Weegink CJ, et al. Rapid decline of viral RNA in hepatitis C patients treated with VX-950: a phase Ib, placebo-controlled, randomized study. Gastroenterology 2006 Oct; 131: 997–1002
Forestier N, Larrey D, Marcellin P, et al. Antiviral activity of danoprevir (ITMN-191/RG7227) in combination with pegylated interferon α-2a and ribavirin in patients with hepatitis J Infect Dis 2011 Aug; 204: 601–8
Acknowledgements
All authors were employees at Hoffmann-La Roche Inc. at the time this work was completed. Micaela Reddy, Jennifer Fretland and Patrick Smith are current employees at Hoffmann-La Roche Inc. Yuan Chen, Joshua Haznedar and Steven Blotner are current employees at Roche/Genentech. Joshua Haznedar owns stock in Roche. All authors have contributed to the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript. This study was supported by Hoffmann-La Roche, Basel, Switzerland. Support for third-party writing assistance for this manuscript was provided by Hoffmann-La Roche. The authors thank Drs Karin Jorga, Thierry Lave and Li Yu for providing valuable insight during the preparation of this manuscript. Additionally, we thank Dr Petra Goelzer for sharing her knowledge of the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, M.B., Chen, Y., Haznedar, J.Ö. et al. Impact of Low-Dose Ritonavir on Danoprevir Pharmacokinetics. Clin Pharmacokinet 51, 457–465 (2012). https://doi.org/10.2165/11599700-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11599700-000000000-00000