Abstract
Background and Objectives: The pharmacokinetics of some medications may be affected by differences in race and ethnicity, which can lead to suboptimal outcomes. The present study was conducted to assess the single- and multiple-dose pharmacokinetics of saxagliptin in healthy Chinese subjects living in China.
Methods: This was an open-label, 9-day study conducted at the Drug Clinical Trial Center, Peking University Third Hospital, Beijing, China. Sixteen healthy Chinese subjects of both sexes between 21 and 33 years of age were administered saxagliptin 5mg orally on day 1, then once daily on days 3–7. Pharmacokinetic variables for saxagliptin (primary outcome) and its active metabolite, 5-hydroxy saxagliptin (secondary outcome), after single and multiple oral doses of saxagliptin were assessed. Safety was also assessed.
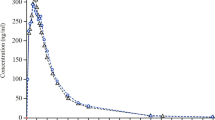
Results: Saxagliptin was absorbed rapidly (median time to reach maximum concentration [tmax]: 0.5 and 1 hour on days 1 and 7, respectively), and its pharmacologically active metabolite, 5-hydroxy saxagliptin, appeared in plasma (median tmax: 1.0 and 1.5 hours, respectively). Plasma exposure to 5-hydroxy saxagliptin was approximately 2- to 3-fold higher than exposure to saxagliptin. Plasma concentration-time profiles for saxagliptin and 5-hydroxy saxagliptin were similar on days 1 and 7, with no evidence of drug accumulation on repeated dosing. The elimination half-lives (t1/2) for saxagliptin and 5-hydroxy saxagliptin were approximately 3 and 4 hours, respectively, with renal excretion as the primary route of elimination. After single and multiple dosing, 54.48% and 52.60%, respectively, of the administered saxagliptin dose was recovered in urine as unchanged drug or 5-hydroxy saxagliptin. Saxagliptin was generally well tolerated. Six (37.5%) subjects experienced an adverse event (AE). All AEs were mild in intensity and judged by the investigator as not related to the study medication. There were no deaths, serious AEs, discontinuations due to AEs, or other clinically significant AEs during this study.
Conclusion: Saxagliptin 5 mg (single dose and once-daily doses for 5 days) was generally well tolerated; the pharmacokinetics of saxagliptin and 5-hydroxy saxagliptin in healthy Chinese subjects were consistent with previous assessments in the saxagliptin clinical development program.
Trial Registration: ClinicalTrials.gov identifier: NCT00770302.






Similar content being viewed by others
References
Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract 2006 Nov; 60 (11): 1454–70
Kirby M, Yu DMT, O’Connor SP, et al. Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition. Clin Sci 2010; 118: 31–41
Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest 2007 Jan; 117 (1): 24–32
Deacon CF. Therapeutic strategies based on glucagon-like peptide 1. Diabetes 2004 Sep; 53 (9): 2181–9
Deacon CF, Holst JJ. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther 2009 May; 26 (5): 488–99
Gallwitz B. Saxagliptin, a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. IDrugs 2008 Dec; 11 (12): 906–17
Boulton DW, Geraldes M. Safety, tolerability, pharmacokinetics and pharmacodynamics of once-daily oral doses of saxagliptin for 2 weeks in type 2 diabetic and healthy subjects [abstract]. Diabetes 2007; 56 Suppl. 1: 606P
Rosenstock J, Aguilar-Salinas C, Klein E, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin 2009 Oct; 25 (10): 2401–11
Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab 2008 May; 10 (5): 376–86
Jadzinsky M, Pfutzner A, Paz-Pacheco E, et al. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 2009 Jun; 11 (6): 611–22
Pfützner A, Paz-Pacheco E, Allen E, et al. Initial combination therapy with saxagliptin and metformin provides sustained glycaemic control and is well tolerated for up to 76 weeks. Diabetes Obes Metab 2011; 13 (6): 567–76
cheen AJ, Charpentier G, Ostgren CJ, et al. Efficacy and safety of saxagliptin in combination with metformin compared with sitagliptin in combination with metformin in adult patients with type 2 diabetes mellitus. Diabetes Metab Res Rev 2010 Oct; 26 (7): 540–9
DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care 2009 Sep; 32 (9): 1649–55
Stenlof K, Raz I, Neutel J, et al. Saxagliptin and metformin XR combination therapy provides glycemic control over 24 hours in patients with T2DM inadequately controlled with metformin. Curr Med Res Opin 2010 Oct; 26 (10): 2355–63
Chacra AR, Tan GH, Apanovitch A, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract 2009 Sep; 63 (9): 1395–406
Hollander P, Li J, Allen E, et al. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab 2009 Dec; 94 (12): 4810–9
Barnett AH, Charbonnel B, Donovan M, et al. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin 2012 Feb 8; 28 (4): 513–23
Nowicki M, Rychlik I, Haller H, et al. Saxagliptin improves glycaemic control and is well tolerated in patients with type 2 diabetes mellitus and renal impairment. Diabetes Obes Metab 2011; 13 (6): 523–32
American Diabetes Association. Standards of medical care in diabetes: 2012. Diabetes Care 2012; 35 Suppl. 1: S11–63
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009 Jan; 32 (1): 193–203
Chacra AR, Tan GH, Ravichandran S, et al. Safety and efficacy of saxagliptin in combination with submaximal sulphonylurea versus up-titrated sulphonylurea over 76 weeks. Diab Vasc Dis Res 2011 Apr; 8 (2): 150–9
Göke B, Gallwitz B, Eriksson J, et al. Saxagliptin is non-inferior to glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: a 52-week randomised controlled trial. Int J Clin Pract 2010 Nov; 64 (12): 1619–31
Onglyza®. Saxagliptin. Princeton (NJ) and Wilmington (DE): Bristol-Myers Squibb Company and AstraZeneca Pharmaceuticals LP; 2011
Scheen AJ. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes Metab 2010 Aug; 12 (8): 648–58
Kirby MS, Dorso C, Wang A, et al. In vitro enzymologic characteristics of saxagliptin, a highly potent and selective DPP4 inhibitor with slow binding characteristics. Clin Chem Lab Med 2008; 46: Abstract A29
Phan VH, Moore MM, McLachlan AJ, et al. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin Drug Metab Toxicol 2009 Mar; 5 (3): 243–57
Shah RR. Pharmacogenetics, ethnic differences in drug response and drug regulation. In: Suarez-Kurtz G, editor. Pharmacogenomics in Admixed Populations. Austin (TX): Landes Bioscience, 2007
World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects [online]. Available from URL: http://www.wma.net/en/30publications/10policies/b3/17c.pdf [Accessed 2011 Apr 19]
US Department of Health and Human Services Food and Drug Administration. Guidance for Industry, E6 Good Clinical Practice: Consolidated Guidance [online]. Available from URL: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073122.pdf [Accessed 2011 Apr 19]
Patel CG, Zhang J, Li L, et al. Effect of a high-fat meal on the pharmacokinetics of saxagliptin in healthy subjects. J Clin Pharmacol 2010 Oct; 50 (10): 1211–6
Gibaldi M, Perrier D. Noncompartmental analysis based on statistical moment theory. Pharmacokinetics. 2nd ed. New York (NY): Marcel Dekker, Inc., 1982: 409–17
Kim K, Johnson JA, Derendorf H. Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol 2004 Oct; 44 (10): 1083–105
Myrand SP, Sekiguchi K, Man MZ, et al. Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther 2008 Sep; 84 (3): 347–61
Cai Z, Christianson AM, Stahle L, et al. Reexamining transaminase elevation in Phase I clinical trials: the importance of baseline and change from baseline. Eur J Clin Pharmacol 2009 Oct; 65 (10): 1025–35
Pan CY, Yang W, Tou C, et al. Efficacy and safety of saxagliptin in drug-naive Asian patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Metab Res Rev 2012 Mar; 28 (3): 268–75
Yang W, Pan CY, Tou C, et al. Efficacy and safety of saxagliptin added to metformin in Asian people with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Res Clin Pract 2011 Nov; 94 (2): 217–24
Acknowledgements
The authors gratefully acknowledge the staff of the Phase I Unit at Peking University Third Hospital in Beijing, China, including Dr Zhenhua Yang for medical assistance, Yifang Zhang, head nurse, for medical support and unit management, and Shuang Zhang for medical assistance. Chemical analysis was conducted by WuXi AppTec, Shanghai, China. Medical writing and editorial assistance with language, consistency of style, and general editing services for this manuscript was provided by Paul Ruest, PhD, and Jessica Uychich, Quintiles Medical Communications, Parsippany, NJ, USA.
Publication support for this manuscript was provided by AstraZeneca and Bristol-Myers Squibb. AstraZeneca and Bristol-Myers Squibb designed and sponsored the current study. June Zhao was involved in design of the study. All of the authors contributed to the conduct, data analysis/interpretation, and independently drafted, critically revised and approved the final manuscript.
Haiyan Li and Li Yang are employees of Peking University Third Hospital. Conrad Tou and June Zhao are employees of AstraZeneca. Chirag Patel was an employee of Bristol-Myers Squibb at the time this study was conducted and the manuscript was written and initially submitted for publication. None of the authors have anything further to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Yang, L., Tou, C.K.P. et al. Pharmacokinetic Study of Saxagliptin in Healthy Chinese Subjects. Clin Drug Investig 32, 465–473 (2012). https://doi.org/10.2165/11598760-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11598760-000000000-00000