Abstract
Background: Previous work has demonstrated the efficacy and safety of fesoterodine in older and younger subjects with overactive bladder (OAB) symptoms. The effect of long-term fesoterodine treatment in different age groups has not been assessed.
Objective: The aim was to determine the impact of age on the safety, tolerability and efficacy of long-term treatment with fesoterodine 8 mg in subjects with OAB syndrome.
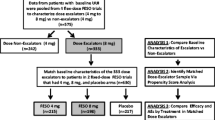
Methods: This was a pooled analysis of two identically designed open-label extensions of 12-week, randomized, double-blind, placebo-controlled studies. The setting was urology and general practice offices. Subjects who participated in the 12-week, double-blind studies and opted to continue long-term, open-label treatment with fesoterodine were included. Subjects were initiated on fesoterodine 8 mg/day at open-label baseline. After 1 month, subjects could elect dose reduction to 4 mg/day and subsequent re-escalation to 8 mg; each was permitted once annually. Maximal duration of open-label treatment ranged from 24 to 36 months. Discontinuations, subject-reported treatment tolerance, and efficacy (3-day diaries) were assessed at open-label baseline and months 1, 4, 8, 12 and 24.
Results: A total of 890 subjects were treated (age <45 years, n = 140; 45–64 years, n = 444; 65–74 years, n = 208; ≥75 years, n = 98); 49% continued treatment for ≥24 months (age <45 years, 43%; 45–64 years, 54%; 65–74 years, 50%; ≥75 years, 37%). Seventy-seven percent of subjects remained on fesoterodine 8mg throughout treatment; this rate was highest among subjects aged ≥75 years (age <45 years, 72%; 45–64 years, 77%; 65–74 years, 73%; ≥75 years, 87%). Approximately 80% of continuing subjects were receiving fesoterodine 8 mg at each visit after open-label baseline up to 36 months. No new or un-expected safety signals were observed in any age group. Most subjects reported ‘good’ or ‘excellent’ treatment tolerance throughout the study (age <45 years, ≥90%; 45–64 years, ≥93%; 65–74 years, ≥85%; ≥75 years, ≥86%). Dry mouth, the most commonly reported treatment-emergent adverse event, was lowest among subjects aged ≥75 years (age <45 years, 31%; 45–64 years, 30%; 65–74 years, 32%; ≥75 years, 26%). Rates of discontinuation due to dry mouth were low in all age groups. Significant improvements in all diary variables, including urgency urinary incontinence episodes per 24 hours, micturitions per 24 hours, urgency episodes per 24 hours, and mean voided volume per micturition, observed between double-blind baseline and open-label baseline were sustained or increased during open-label treatment in the overall population and all age groups.
Conclusions: Long-term fesoterodine (administered primarily as 8 mg) was well tolerated and associated with sustained improvements in OAB symptoms, irrespective of age.






Similar content being viewed by others
References
Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 2002; 21(2): 167–78
Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010; 29(1): 4–20
Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006 Dec; 50(6): 1306–15
Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003 May; 20(6): 327–36
Wyman JF, Burgio KL, Newman DK. Practical aspects of lifestyle modifications and behavioural interventions in the treatment of overactive bladder and urgency urinary incontinence. Int J Clin Pract 2009 Aug; 63(8): 1177–91
Andersson KE, Chapple CR, Cardozo L, et al. Pharmacological treatment of overactive bladder: report from the International Consultation on Incontinence. Curr Opin Urol 2009 May 14; 19(4): 380–94
Chapple C, Van Kerrebroeck P, Tubaro A, et al. Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Eur Urol 2007 Oct; 52(4): 1204–12
Nitti VW, Dmochowski R, Sand PK, et al. Efficacy, safety and tolerability of fesoterodine for overactive bladder syndrome. J Urol 2007 Dec; 178(6): 2488–94
Khullar V, Rovner ES, Dmochowski R, et al. Fesoterodine dose response in subjects with overactive bladder syndrome. Urology 2008 May; 71(5): 839–43
Chapple C, Van Kerrebroeck P, Juenemann K, et al. Comparison of fesoterodine and tolterodine in subjects with overactive bladder. BJU Int 2008; 102(9): 1128–32
Herschorn S, Swift S, Guan Z, et al. Comparison of fesoterodine and tolterodine extended release for the treatment of overactive bladder: a head-to-head placebo-controlled trial. BJU Int 2010 Jan; 105(1): 58–66
Kaplan S, Schneider T, Foote J, et al. Superior efficacy of fesoterodine over tolterodine extended release with rapid onset: a prospective, head-to-head, placebo-controlled trial. BJU Int 2011; 107(9): 1432–40
Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 2009; 15(9): 728–40
Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 2009 May; 105(9): 1276–82
Irwin DE, Abrams P, Milsom I, et al. Understanding the elements of overactive bladder: questions raised by the EPIC study. BJU Int 2008 Jun; 101(11): 1381–7
Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 2004 Jan; 57(1): 6–14
Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther 2007 Jul; 82(1): 87–96
Kraus SR, Ruiz-Cerdá JL, Martire D, et al. Efficacy and tolerability of fesoterodine in older and younger subjects with overactive bladder. Urology 2010; 76: 1350–7
Irwin DE, Milsom I, Kopp Z, et al. Symptom bother and health care-seeking behavior among individuals with overactive bladder. Eur Urol 2008 May; 53(5): 1029–37
Coyne KS, Payne C, Bhattacharyya SK, et al. The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Value Health 2004 Jul–Aug; 7(4): 455–63
Coyne KS, Sexton CC, Irwin DE, et al. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 2008 Jun; 101(11): 1388–95
Irwin DE, Milsom I, Kopp Z, et al. Impact of overactive bladder symptoms on employment, social interactions and emotional well-being in six European countries. BJU Int 2006 Jan; 97(1): 96–100
Michel MC, Schneider T, Krege S, et al. Does gender or age affect the efficacy and safety of tolterodine? J Urol 2002 Sep; 168(3): 1027–31
Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004 Apr; 99(4): 750–9
Haab F, Corcos J, Siami P, et al. Long-term treatment with darifenacin for overactive bladder: results of a 2-year, open-label extension study. BJU Int 2006 Nov; 98(5): 1025–32
Haab F, Cardozo L, Chapple C, et al. Long-term open-label solifenacin treatment associated with persistence with therapy in patients with overactive bladder syndrome. Eur Urol 2005 Mar; 47(3): 376–84
Abrams P, Malone-Lee J, Jacquetin B, et al. Twelve-month treatment of overactive bladder: efficacy and tolerability of tolterodine. Drugs Aging 2001; 18(7): 551–60
Dmochowski RR, Peters KM, Morrow JD, et al. Randomized, double-blind, placebo-controlled trial of flexible-dose fesoterodine in subjects with overactive bladder. Urology 2010; 75: 62–8
Wyndaele JJ, Goldfischer ER, Morrow JD, et al. Effects of flexible-dose fesoterodine on overactive bladder symptoms and treatment satisfaction: an open-label study. Int J Clin Pract 2009 Apr; 63(4): 560–7
Chapple C, DuBeau C, Ebinger U, et al. Darifenacin treatment of patients >or= 65 years with overactive bladder: results of a randomized, controlled, 12-week trial. Curr Med Res Opin 2007 Oct; 23(10): 2347–58
Griebling TL, Kraus SR, Richter HE, et al. Tolterodine extended release is well tolerated in older subjects. Int J Clin Pract 2009 Aug; 63(8): 1198–204
Malone-Lee JG, Walsh JB, Maugourd MF. Tolterodine: a safe and effective treatment for older patients with overactive bladder. J Am Geriatr Soc 2001 Jun; 49(6): 700–5
Wagg A, Wyndaele JJ, Sieber P. Efficacy and tolerability of solifenacin in elderly subjects with overactive bladder syndrome: a pooled analysis. Am J Geriatr Pharmacother 2006 Mar; 4(1): 14–24
Zinner NR, Mattiasson A, Stanton SL. Efficacy, safety, and tolerability of extended-release once-daily tolterodine treatment for overactive bladder in older versus younger patients. J Am Geriatr Soc 2002 May; 50(5): 799–807
van Leeuwen JHS, Castro R, Busse M, et al. The placebo effect in the pharmacologic treatment of patients with lower urinary tract symptoms. Eur Urol 2006 May 19; 50(3): 440–53
Acknowledgements
Funding for both extension studies was provided by Schwarz BioSciences GmbH and Pfizer Inc. Schwarz BioSciences GmbH participated in the design and conduct of the studies; Pfizer Inc. was involved in data analysis and manuscript preparation. Editorial assistance was provided by Nancy Sheridan and Colin Mitchell, PhD, from Complete Healthcare Communications, Inc., and was funded by Pfizer Inc.
Author’s Contributions: All authors had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors participated in study concept and design, interpretation of data, drafting, and revising the article, and all have given final approval for the manuscript to be published. Other author contributions were as follows: acquisition of data: P.K.S., S.R.K. and J.H.; statistical analysis: M.C.
Conflicts of Interest: P.K.S. has been an advisor and speaker for Allergan, Astellas, GlaxoSmithKline, Ortho, Pfizer, Teva and Watson, and has received research grants from Allergan, Antares, Contura, Bioform, Boston Scientific, Ortho, Pfizer and Watson. J.H. has been an advisor and speaker for Allergan, Astellas, Pfizer and Uroplasty, and has received grants from Astellas, Medtronic, Pfizer and Pohl Boskamp. S.R.K. has been a consultant and lecturer for Pfizer and Laborie and has received research grants from Pfizer and the National Institute of Diabetes and Digestive and Kidney Diseases. M.C. and Z.G. are employees of Pfizer. S.B. was an employee of Pfizer at the time this study was conducted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sand, P.K., Heesakkers, J., Kraus, S.R. et al. Long-Term Safety, Tolerability and Efficacy of Fesoterodine in Subjects with Overactive Bladder Symptoms Stratified by Age. Drugs Aging 29, 119–131 (2012). https://doi.org/10.2165/11597970-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11597970-000000000-00000