Abstract
Background: Granulocyte-colony stimulating factor (G-CSF) reduces the risk of severe neutropenia associated with chemotherapy, but its cost implications following chemotherapy are unknown.
Objective: Our objective was to examine associations between G-CSF use and medical costs after initial adjuvant chemotherapy in early-stage (stage I–III) breast cancer (ESBC).
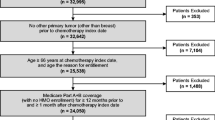
Methods: Women diagnosed with ESBC from 1999 to 2005, who had an initial course of chemotherapy beginning within 180 days of diagnosis and including ≥1 highly myelosuppressive agent, were identified from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database.
Medicare claims were used to describe the initial chemotherapy regimen according to the classes of agents used: anthracycline ([A]: doxorubicin or epirubicin); cyclophosphamide (C); taxane ([T]: paclitaxel or docetaxel); and fluorouracil (F). Patients were classified into four study groups according to their G-CSF use: (i) primary prophylaxis, if the first G-CSF claim was within 5 days of the start of the first chemotherapy cycle; (ii) secondary prophylaxis, if the first claim was within 5 days of the start of the second or subsequent cycles; (iii)G-CSF treatment, if the first claim occurred outside of prophylactic use; and (iv) no G-CSF. Patients were described by age, race, year of diagnosis, stage, grade, estrogen (ER) and progesterone (PR) receptor status, National Cancer Institute (NCI) Co-morbidity Index, chemotherapy regimen and G-CSF use.
Total direct medical costs ($US, year 2009 values) to Medicare were estimated from 4 weeks after the last chemotherapy administration up to 48 months. Medical costs included those for ESBC treatment and all other medical services received after chemotherapy.
Least squares regression, using inverse probability weighting (IPW) to account for censoring within the cohort, was used to evaluate adjusted associations between G-CSF use and costs. Results: A total of 7026 patients were identified, with an average age of 72 years, of which 63% had stage II disease, and 59% were ER and/or PR positive. Compared with no G-CSF, those receiving G-CSF primary prophylaxis were more likely to have stage III disease (30% vs 16%; p < 0.0001), to be diagnosed in 2003–5 (87% vs 26%; p < 0.0001), and to receive dose-dense AC-T (26% vs 1%; p < 0.0001), while they were less likely to receive an F-based regimen (12% vs 42%; p < 0.0001).
Overall, the estimated average direct medical cost over 48months after initial chemotherapy was $US42 628. In multivariate analysis, stage II or III diagnosis (compared with stage I),NCI Co-morbidity Index score 1 or ≥2 (compared with 0), or FAC or standard AC-T (each compared with AC) were associated with significantly higher IPW 48-month costs. Adjusting for patient demographic and clinical factors, costs in the G-CSF primary prophylaxis group were not significantly different from those not receiving primary prophylaxis (the other three study groups combined). In an analysis that included four separate study groups, G-CSF treatment was associated with significantly greater costs (incremental cost = $US2938; 95% CI 285, 5590) than no G-CSF.
Conclusions: Direct medical costs after initial chemotherapy were not statistically different between those receiving G-CSF primary prophylaxis and those receiving no G-CSF, after adjusting for potential confounders.










Similar content being viewed by others
References
Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer 2006; 42 (15): 2433–53
Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 2006; 24 (19): 3187–205
National Comprehensive Cancer Network. Practice guidelines in oncology v.1.2010: myeloid growth factors [online]. Available with user ID and password from URL: http://www.nccn.org/professionals/physician_gls/PDF/myeloid_growth.pdf. [Accessed 2010 Oct 1]
Greil R, Psenak O, Roila F. Hematopoietic growth factors: ESMO recommendations for the applications. Ann Oncol 2008; 19 Suppl. 2: ii116–8
Carrato A, Paz-Ares Rodriguez L, Rodriguez Lescure A, et al. Spanish Society of Medical Oncology consensus for the use of haematopoietic colony-stimulating factors in cancer patients. Clin Transl Oncol 2009; 11 (7): 446–54
Aapro M, Crawford J, Kamioner D. Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colony-stimulating factors: where are we now? Support Care Cancer 2010; 18 (5): 529–41
Martin M, Lluch A, Segui MA, et al. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte colony stimulating factor to the TAC regimen. Ann Oncol 2006; 17 (8): 1205–12
von Minckwitz G, Kümmel S, du Bois A, et al. Pegfilgrastim +/- ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer: results from the GEPARTRIO study. Ann Oncol 2008; 19 (2): 292–8
Piedbois P, Serin D, Priou F, et al. Dose-dense adjuvant chemotherapy in node-positive breast cancer: docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Ann Oncol 2007; 18 (1): 52–7
Wildiers H, Dirix L, Neven P, et al. Delivery of adjuvant sequential dose-dense FEC-Doc to patients with breast cancer is feasible, but dose reductions and toxicity are dependent on treatment sequence. Breast Cancer Res Treat 2009; 114 (1): 103–12
Bosly A, Bron D, Van Hoof A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol 2007; 87 (4): 277–83
Bonadonna G, Valagussa P, Moliterni A, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in nodepositive breast cancer: the results of 20 years of follow-up. N Engl J Med 1995; 332 (14): 901–6
Lee KW, Kim DY, Yun T, et al. Doxorubicin-based chemotherapy for diffuse large B-cell lymphoma in elderly patients: comparison of treatment outcomes between young and elderly patients and the significance of doxorubicin dosage. Cancer 2003; 98 (12): 2651–6
Kwak LW, Halpern J, Olshen RA, et al. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol 1990; 8 (6): 963–77
Chirivella I, Bermejo B, Insa A, et al. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat 2009; 114 (3): 479–84
Pettengell R, Schwenkglenks M, Bosly A. Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol 2008; 87 (5): 429–30
Earl HM, Hiller L, Dunn JA, et al. NEAT: National Epirubicin Adjuvant Trial: toxicity, delivered dose intensity and quality of life. Br J Cancer 2008; 99 (8): 1226–31
Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 2008; 100 (9): 630–41
Stokes ME, Thompson D, Montoya EL, et al. Ten-year survival and cost following breast cancer recurrence: estimates from SEER-Medicare data. Value Health 2008; 11 (2): 213–20
Eldar-Lissai A, Cosler LE, Culakova E, et al. Economic analysis of prophylactic pegfilgrastim in adult cancer patients receiving chemotherapy. Value Health 2008; 11 (2): 172–9
Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw 2009; 7 (1): 99–108
Warren JL, Brown ML, Fay MP, et al. Costs of treatment for elderly women with early-stage breast cancer in fee-forservice settings. J Clin Oncol 2002; 20 (1): 307–16
National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat database: Incidence-SEER 17 Regs Limited-Use+Hurricane Katrina Impacted Louisiana Cases, Nov 2008 Sub (1973-2006 varying)-Linked To County Attributes-Total U.S., 1969-2006 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2009, based on the November 2008 submission [online]. Available from URL: http://www.seer.cancer.gov [Accessed 2010 Jun 1]
National Cancer Institute. SEER-Medicare: how the SEER & Medicare data are linked. Bethesda (MD): National Cancer Institute, 2010 [online]. Available from URL: http://healthservices.cancer.gov/seermedicare/overview/linked.html [Accessed 2010 May 25]
Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol 2007; 17 (8): 584–90
Fritz A, Ries L, eds. The SEER program code manual. 3rd ed. Bethesda (MD): Cancer Statistics Branch, Surveillance Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Public Health Service, US Dept of Health and Human Services, 1998 [online]. Available from URL: http://seer.cancer.gov/manuals/codeman.pdf [Accessed 2010 Oct 5]
National Cancer Institute: Health Services and Economics-SEER-Medicare Programming Support. 2010 [online]. Available from URL: http://healthservices.cancer.gov/seermedicare/program/remove.ruleout.dxcodes.macro.txt [Accessed 2010 Aug 27]
National Cancer Institute: Health Services and Economics-SEER-Medicare Programming Support (Charlson Comorbidity). 2010 [online]. Available from URL: http://healthservices.cancer.gov/seermedicare/program/charlson.comorbidity.macro.txt [Accessed 2010 Aug 27]
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40 (5): 373–83
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45 (6): 613–9
Romano PS, Roos LL, Luft HS, et al. A comparison of administrative versus clinical data: coronary artery bypass surgery as an example. Ischemic Heart Disease Patient Outcomes Research Team. J Clin Epidemiol 1994; 47 (3): 249–60
National Comprehensive Cancer Network. NCCN guidelines for patients: breast cancer. Fort Washington (PA): NCCN [online]. Available from URL: http://www.nccn.com/images/patient-guidelines/pdf/breast.pdf [Accessed 2010 Oct 5]
Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika 2000; 87 (2): 329–43
Lin DY. Linear regression analysis of censored medical costs. Biostatistics 2000; 1 (1): 35–47
Efron B, Tibshirani RJ. An introduction to the bootstrap (monographs on statistics and applied probability). Boca Raton (FL): Chapman & Hall/CRC, 1994
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365 (9472): 1687–717
Siegel J. FDA approval letter: pegfilgrastim. Rockville (MD) [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4185B1_03_08-FDATab5a.pdf [Accessed 2010 Oct 5]
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235 (4785): 177–82
Hudis CA. Trastuzumab: mechanism of action and use in clinical practice. N Engl J Med 2007; 357 (1): 39–51
National Cancer Institute. FDA approval for trastuzumab. Bethesda (MD): NCI, 2010 [online]. Available from URL: http://www.cancer.gov/cancertopics/druginfo/fda-trastuzumab [Accessed 2010 Oct 5]
Basu A, Manning WG. Estimating lifetime or episodeof-illness costs under censoring. Health Econ 2010; 19 (9): 1010–28
Imbens GW. Nonparametric estimation of average treatment effects under exogeneity: a review. Rev Econ Stat 2004; 86 (1): 4–29
Cattaneo MD, Crump RK, Jansson M. Bootstrapping density-weighted average derivatives. Federal Reserve Bank of New York Staff Reports 2010 May; 452
Acknowledgements
Funding for conduct of the study and preparation of the paper was provided by Amgen Inc. Richard L. Barron, Victoria M. Chia and Jason C. Legg, who are employees of Amgen Inc., participated in the design and conduct of the study, as well as the preparation and review of the manuscript. However, by contract, Outcomes Insights, Inc. retained the right to publish the findings independent of the sponsor. Robert I. Griffiths, Michelle L. Gleeson and Mark D. Danese are employees of Outcomes Insights, Inc., which received funding from Amgen Inc. to conduct the study. Richard L. Barron, Victoria M. Chia and Jason C. Legg are employees of and shareholders in Amgen Inc. Anthony O’Hagan is a consultant to Outcomes Insights, Inc. Gary H. Lyman has received grant support from Amgen Inc.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute (NCI); the Office of Research, Development and Information, Centers for Medicare and Medicaid Services (CMS); Information Management Services (IMS), Inc.; and the SEER Program tumour registries in the creation of the SEER-Medicare database.
The authors would like to acknowledge the editorial assistance of Jennifer Deuson and Brittany Masson in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Griffiths, R.I., Barron, R.L., Gleeson, M.L. et al. Granulocyte-Colony Stimulating Factor Use and Medical Costs after Initial Adjuvant Chemotherapy in Older Patients with Early-Stage Breast Cancer. PharmacoEconomics 30, 103–118 (2012). https://doi.org/10.2165/11589440-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11589440-000000000-00000