Abstract
Background
Smoking is the leading cause of preventable death in the US. While one in five individuals smoke, and 70% of these indicate a desire to quit, <5% of unaided quit attempts succeed. Cessation aids can double or triple the odds of successfully quitting. Models of smoking-cessation behaviour can elucidate the implications of individual abstinence patterns to allow better tailoring of quit attempts to an individual’s characteristics.
Objective
The objectives of this study were to develop and validate a discrete-event simulation (DES) to evaluate the benefits of smoking abstinence using data from the pooled pivotal clinical trials of varenicline versus bupropion or placebo for smoking cessation and to provide a foundation for the development of a lifetime smoking-cessation model.
Methods
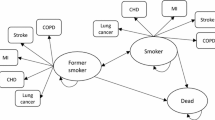
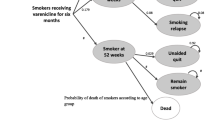
The DES model simulated the outcome of a single smoking-cessation attempt over 1 year, in accordance with the clinical trial timeframes. Pharmaceutical costs were assessed from the perspective of a healthcare payer. The model randomly sampled patient profiles from the pooled varenicline clinical trials. All patients were physically and mentally healthy adult smokers who were motivated to quit abruptly. The model allowed for comparisons of up to five distinct treatment approaches for smoking cessation. In the current analyses, three interventions corresponding to the clinical trials were evaluated, which included brief counselling plus varenicline 1.0 mg twice daily (bid) or bupropion SR 150mg bid versus placebo (i.e. brief counselling only). The treatment periods in the clinical trials were 12 weeks (target quit date: day 8), with a 40-week non-treatment follow-up, and counselling continuing over the entire 52-week period in all treatment groups. The main outcome modelled was the continuous abstinence rate (CAR; defined as complete abstinence from smoking and confirmed by exhaled carbon monoxide ≤10 ppm) at end of treatment (weeks 9–12) and long-term follow-up (weeks 9–52), and total time abstinent from smoking over the course of 52 weeks. The model also evaluated costs and cost-effectiveness outcomes.
Results
For the varenicline, bupropion and placebo cohorts, respectively, the model predicted CARs for weeks 9–12 of 44.3%, 30.4% and 18.6% compared with observed rates of 44.0%, 29.7% and 17.7%; over weeks 9–52, predicted CARs in the model compared with observed rates in the pooled clinical studies were 22.9%, 16.4% and 9.4% versus 22.4%, 15.4% and 9.3%, respectively. Total mean abstinence times accrued in the model varenicline, bupropion and placebo groups, respectively, were 3.6, 2.6 and 1.5 months and total pharmaceutical treatment costs were $US261, $US442 and $US0 (year 2008 values) over the 1-year model period. Using cost per abstinent-month achieved as a measure of cost effectiveness, varenicline dominated bupropion and yielded an incremental cost-effectiveness ratio of $US124 compared with placebo.
Conclusion
The model accurately replicated abstinence patterns observed in the clinical trial data using individualized predictions and indicated that varenicline was more effective and may be less costly than bupropion. This simulation incorporated individual predictions of abstinence and relapse, and provides a framework for lifetime modelling that considers multiple quit attempts over time in diverse patient populations using a variety of quit attempt strategies.
Similar content being viewed by others
References
Centers for Disease Control and Prevention (CDC). Annual smoking-attributable mortality, years of potential life lost, and economic costs: United States, 1995–1999. MMWR Morb Mortal Wkly Rep 2002 Apr 12; 51 (14): 300–3
Centers for Disease Control and Prevention (CDC). State-specific smoking-attributable mortality and years of potential life lost: United States, 2000–2004. MMWR Morb Mortal Wkly Rep 2009 Jan 23; 58 (2): 29–33
Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses: United States, 2000–2004. MMWR Morb Mortal Wkly Rep 2008 Nov 14; 57 (45): 1226–8
US Department of Health and Human Services. Office of the Surgeon General. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. 2006 Jun 27 [online]. Available from URL: http://www.surgeongeneral.gov/library/secondhandsmoke/ [Accessed 2010 Sep 6]
Kaper J, Wagena EJ, Willemsen MC, et al. Reimbursement for smoking cessation treatment may double the abstinence rate: results of a randomized trial. Addiction 2005 Jul; 100 (7): 1012–20
Centers for Disease Control and Prevention (CDC). Cigarette smoking among adults: United States, 2006. MMWR 2007 Nov 9; 56 (44): 1157–61
Taylor T, Lader D, Bryant A, et al. Smoking-related behaviour and attitudes, 2005: a report on research using the National Statistics Omnibus Survey produced on behalf of the Department of Health and the Information Centre for Health and Social Care. 2006 [online]. Available from URL: http://www.statistics.gov.uk/downloads/theme_health/Smoking2005.pdf [Accessed 2010 Sep 6]
Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004 Jan; 99 (1): 29–38
Nides M. Update on pharmacologic options for smoking cessation treatment. Am J Med 2008 Apr; 121 (4 Suppl. 1): S20–31
Hajek P, Stead LF, West R, et al. Relapse prevention interventions for smoking cessation. Cochrane Database System Rev 2009 Jan 21; (1): CD003999
Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2007 Jan 24; (1): CD006103
Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: quick reference guide for clinicians, 2008 update. Rockville (MD): US Department of Health and Human Services, 2009 Apr [online]. Available from URL: http://www.ahrq.gov/clinic/tobacco/tobaqrg.pdf [Accessed 2010 Sep 6]
Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007 Jan 24; (1): CD000031
Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2008 Jan 23; (1): CD000146
Armour BS, Finkelstein EA, Fiebelkorn IC. State-level Medicaid expenditures attributable to smoking. Prev Chronic Dis 2009 Jul; 6 (3): A84
Foulds J, Burke M, Steinberg M, et al. Advances in pharmacotherapy for tobacco dependence. Expert Opin Emerg Drugs 2004 May; 9 (1): 39–53
Ranney L, Melvin C, Lux L, et al. Systematic review: smoking cessation intervention strategies for adults and adults in special populations. Ann Int Med 2006 Dec 5; 145 (11): 845–56
Joyce GF, Niaura R, Maglione M, et al. The effectiveness of covering smoking cessation services for medicare beneficiaries. Health Serv Res 2008 Dec; 43 (6): 2106–23
Centers for Disease Control and Prevention (CDC). State medicaid coverage for tobacco-dependence treatments: United States, 2009. MMWR Morb Mortal Wkly Rep 2010 Oct 22; 59 (41): 1340–3
Gonzales D, Rennard SI, Nides M, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006 Jul 5; 296 (1): 47–55
Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006 Jul 5; 296(1): 56–63
Caro JJ. Pharmacoeconomic analyses using discrete event simulation. Pharmacoeconomics 2005; 23 (4): 323–32
Caro JJ, Möller J, Getsios D. Discrete event simulation: the preferred technique for health economic evaluations? Value Health 2010 Dec; 13 (8): 1056–60
Simpson KN, Strassburger A, Jones WJ, et al. Comparison of Markov model and discrete-event simulation techniques for HIV. Pharmacoeconomics 2009; 27 (2): 159–65
Weinstein MC. Recent developments in decision-analytic modelling for economic evaluation. Pharmacoeconomics 2006; 24 (11): 1043–53
Bolin K, Wilson K, Benhaddi H, et al. Cost-effectiveness of varenicline compared with nicotine patches for smoking cessation: results from four European countries. Eur J Public Health 2009 Dec; 19 (6): 650–4
Hoogendoorn M, Welsing P, Rutten-van Molken MP. Cost-effectiveness of varenicline compared with bupropion, NRT, and nortriptyline for smoking cessation in the Netherlands. Curr Med Res Opin 2008 Jan; 24 (1): 51–61
Howard P, Knight C, Boler A, et al. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO simulation model: application to a population of US adult smokers. Pharmacoeconomics 2008; 26 (6): 497–511
Knight C, Howard P, Baker CL, et al. The cost-effectiveness of an extended course (12+12 weeks) of varenicline compared with other available smoking cessation strategies in the United States: an extension and update to the BENESCO model. Value Health 2010 Mar; 13 (2): 209–14
Law AM, Kelton WD. Simulations modeling and analysis. Boston (MA): McGraw-Hill, 2000
Babad H, Sanderson C, Naidoo B, et al. The development of a simulation model of primary prevention strategies for coronary heart disease. Health Care Manag Sci 2002 Nov; 5 (4): 269–74
Bozzette SA, Boer R, Bhatnagar V, et al. A model for a smallpox-vaccination policy. N Eng J Med 2003 Jan 30; 348 (5): 416–25
Cahill W, Render M. Dynamic simulation modeling of ICU bed availability. In: Farrington PA, Nembhard HB, Sturrock DT, et al., editors. Proceedings of the 1999 Winter Simulation Conference (WSC’99); 1999 Dec 5–8; Phoenix (AZ). Piscataway (NJ): IEEE Standards Office, 1999: 1573–6
Caro J, Ward A, Moller J. Modelling the health benefits and economic implications of implanting dual-chamber vs. single-chamber ventricular pacemakers in the UK. Europace 2006 Jun; 8 (6): 449–55
Cooper K, Davies R, Roderick P, et al. The development of a simulation model of the treatment of coronary heart disease. Health Care Manag Sci 2002 Nov; 5 (4): 259–67
Davies R, Roderick P, Canning C, et al. The evaluation of screening policies for diabetic retinopathy using simulation. Diabet Med 2002 Sep; 19 (9): 762–70
Guest JF, Cookson RF. Cost of schizophrenia to UK society: an incidence-based cost-of-illness model for the first 5 years following diagnosis. Pharmacoeconomics 1999 Jun; 15(6): 597–610
Huybrechts KF, Caro JJ, Wilson DA, et al. Health and economic consequences of sevelamer use for hyperphos-phatemia in patients on hemodialysis. Value Health 2005 Sep–Oct; 8 (5): 549–61
Kelton WD, Sadowski RP, Sadowski DA. Simulation with ARENA. 3rd ed. Boston (MA): McGraw-Hill, 2003
McEwan P, Peters JR, Bergenheim K, et al. Evaluation of the costs and outcomes from changes in risk factors in type 2 diabetes using the Cardiff stochastic simulation cost-utility model (DiabForecaster). Curr Med Res Opin 2006 Jan; 22 (1): 121–9
Ward A, Bozkaya D, Fleischmann J, et al. Modeling the economic and health consequences of managing chronic osteoarthritis pain with opioids in Germany: comparison of extended-release oxycodone and OROS hydromorphone. Curr Med Res Opin 2007 Oct; 23 (10): 2333–45
Warner KE, Smith RJ, Smith DG, et al. Health and economic implications of a work-site smoking-cessation program: a simulation analysis. J Occup Environ Med 1996 Oct; 38 (10): 981–92
Arias E. United States life tables, 2004. Natl Vital Stat Rep 2007; 56 (9): 1–39
Nides M, Glover ED, Reus VI, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. Am J Health Behav 2008 Nov–Dec; 32 (6): 664–75
Doll R, Hill AB. The mortality of doctors in relation to their smoking habits: a preliminary report. 1954. BMJ 2004 Jun 26; 328 (7455): 1529–33; discussion 1533
Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004 Jun 26; 328 (7455): 1519
Hoogenveen RT, van Baal PH, Boshuizen HC, et al. Dynamic effects of smoking cessation on disease incidence, mortality and quality of life: the role of time since cessation. Cost Eff Resour Alloc 2008 Jan 11; 6: 1
Caro JJ, Getsios D, Möller J. Regarding probabilistic analysis and computationally expensive models: necessary and required? Value Health 2007 Jul–Aug; 10 (4): 317–8; author reply 319
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xenakis, J.G., Kinter, E.T., Ishak, K.J. et al. A Discrete-Event Simulation of Smoking-Cessation Strategies Based on Varenicline Pivotal Trial Data. Pharmacoeconomics 29, 497–510 (2011). https://doi.org/10.2165/11589230-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11589230-000000000-00000