Abstract
Background: Corticosteroids are a versatile option for the treatment of mild-to-moderate psoriasis due to their availability in a wide range of potencies and formulations. Occlusion of the corticosteroid is a widely accepted procedure to enhance the penetration of the medication, thereby improving its effectiveness. Betamethasone valerate (BMV) is a moderately potent corticosteroid that is available as a cream, ointment, and lotion. A ready-to-use occlusive dressing, which provides a continuous sustained release of BMV, has been developed for the treatment of psoriasis.
Objective: To evaluate the efficacy and safety of a new BMV 0.1% plaster compared with a BMV 0.1% cream in patients with mild-to-moderate chronic plaque psoriasis.
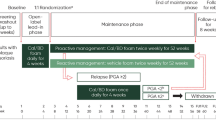
Methods: This was a prospective, randomized, assessor-blind, parallel-group, active-controlled, multicenter, phase III study. Eligible outpatients (aged ≥18 years) with a diagnosis of stable, chronic plaque psoriasis vulgaris with two to four plaques on extensor surfaces of limbs were randomized to receive BMV 0.1% plaster or BMV 0.1% cream for 3–5 weeks; patients with resolution of target plaques then entered a 3-month, treatment-free, follow-up period. The number of patients showing clearing of plaques (remission) at 3 weeks (primary endpoint) and at 5 weeks was independently evaluated from digitized images of target plaques by two blinded assessors, and also assessed by the investigator and patient. Additional endpoints were (i) change from baseline in target plaque size and in Psoriasis Global Assessment (PGA) score, as evaluated by the blinded assessors, investigator, and patient; (ii) change from baseline in symptom (itching, soreness) severity; (iii) treatment satisfaction and ease of use; (iv) clearing and relapse during the follow-up period; and (v) adverse events (AEs).
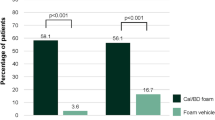
Results: Patients (n = 231) were screened and randomized to treatment with BMV 0.1% plaster (n = 116) and BMV 0.1% cream (n = 115). Significantly more patients achieved clearing after 3 weeks’ treatment with BMV plaster than with BMV cream (Cochran-Mantel-Haenszel test, p < 0.001); this difference was maintained at 5 weeks. The total plaque area decreased to a larger extent for the BMV plaster group compared with the BMV cream group (analysis of covariance [ANCOVA] model, p = 0.017 at week 5). PGA scores were significantly lower after 3 and 5 weeks’ treatment with BMV plaster (ANCOVA model, all p ≤ 0.016 vs BMV cream). Both treatments reduced itching and soreness to a similar degree, and the incidences of relapse during the follow-up period were comparable between treatment groups. There were no significant differences in AEs between treatment groups.
Conclusions: BMV 0.1% plaster is more efficacious than BMV 0.1% cream in the treatment of patients with mild-to-moderate chronic plaque psoriasis in a clinical setting resembling daily clinical practice.
Clinical trial number: ISRCTN68864186
Similar content being viewed by others
References
Greaves MW, Weinstein GD. Treatment of psoriasis. N Engl J Med 1995 Mar 2; 332 (9): 581–8
Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3, guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009 Apr; 60 (4): 643–59
Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007 Jul 21; 370 (9583): 263–71
Mason AR, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev 2009; (2): CD005028
Federman DG, Froelich CW, Kirsner RS. Topical psoriasis therapy. Am Fam Physician 1999 Feb 15; 59 (4): 957–62, 64
Afifi T, de Gannes G, Huang C, et al. Topical therapies for psoriasis: evidencebased review. Can Fam Physician 2005 Apr; 51: 519–25
IBSA Institut Biochimique SA. Betesil 2.25 mg medicated plaster; 2009 [online]. Available from URL: (http://www.ibsa.ch/welcome-intl/productsdermatology-betesil-intl.htm?id=1244) [Accessed 2011 Jan 6]
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP): guideline on clinical investigation of medicinal products indicated for the treatment of psoriasis [online]. Available from URL: (http://www.ema.europa.eu/pdfs/human/ewp/245402en.pdf) [Accessed 2011 Jan 6]
Betneval® 0.1% cream (package insert) [online]. Available from URL: (http://www.gsk.fr/gsk/medicament/notice/Betneval.pdf) [Accessed 2011 Jan 6]
Wilkinson RD, Ohayon M. Therapeutic response to a dermatologic patch and betamethasone valerate 0.1 percent cream in the management of chronic plaques in psoriasis. Cutis 1990 Jun; 45 (6): 468–70
Pacifico A, Daidone R, Peris K. A new formulation of an occlusive dressing containing betamethasone valerate 0.1% in the treatment of mild to moderate psoriasis. J Eur Acad Dermatol Venereol 2006 Feb; 20 (2): 153–7
Actiderm Multi-Center Study Group. A trial of the Actiderm dermatological patch and topical corticosteroids in the treatment of psoriasis vulgaris. Cutis 1990 Jul; 46 (1): 84–8
Broby-Johansen U, Karlsmark T, Petersen LJ, et al. Ranking of the antipsoriatic effect of various topical corticosteroids applied under a hydrocolloid dressing: skin-thickness, blood-flow and colour measurements compared to clinical assessments. Clin Exp Dermatol 1990 Sep; 15 (5): 343–8
Volden G, Kragballe K, Van De Kerkhof PC, et al. Remission and relapse of chronic plaque psoriasis treated once a week with clobetasol propionate occluded with a hydrocolloid dressing versus twice daily treatment with clobetasol propionate alone. J Dermatolog Treat 2001 Sep; 12 (3): 141–4
van der Vleuten CJ, van Vlijmen-Willems IM, de Jong EM, et al. Clobetasol-17 propionate lotion under hydrocolloid dressing (Duoderm ET) once weekly versus unoccluded clobetasol-17-propionate ointment twice daily in psoriasis: an immunohistochemical study on remission and relapse. Arch Dermatol Res 1999 Jul-Aug; 291 (7-8): 390–5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naldi, L., Yawalkar, N., Kaszuba, A. et al. Efficacy and Safety of the Betamethasone Valerate 0.1% Plaster in Mild-to-Moderate Chronic Plaque Psoriasis. Am J Clin Dermatol 12, 191–201 (2011). https://doi.org/10.2165/11539780-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11539780-000000000-00000