Abstract
Background
In Sweden, risperidone long-acting injectable (RLAI) is generally used in a population of schizophrenia patients who are at a high risk of being non-compliant. However, RLAI might also be suitable for use in the general schizophrenia population.
Objectives
To analyse the clinical and economic effects of RLAI in the Swedish treatment practice using a discrete-event simulation (DES) model. Treatment outcomes and direct costs were analysed for both the high-risk non-compliant patient population and the general schizophrenia population.
Methods
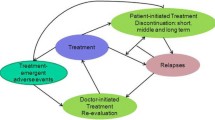
An existing DES model was adapted to simulate the treatment of schizophrenia in Sweden. Model inputs were based on literature research and supplemented with expert opinion. In the high-risk non-compliant schizophrenia population, RLAI was compared with haloperidol LAI. The analysis was built upon differences in symptom reduction, time between relapses, compliance and adverse effect profile between the two drugs. Main outcomes were the predicted incremental (discounted) costs (€) and effects (QALYs). In the general schizophrenia population, RLAI was compared with oral olanzapine. This analysis was built upon differences in compliance and adverse effects between the drugs. Multivariate probabilistic sensitivity analyses (PSA) were carried out to assess the sensitivity of the results of the two analyses.
Results
In the high-risk non-compliant patient population, RLAI was predicted to generate 0.103 QALYs per patient over 3 years while realizing cost savings of €5013 (year 2007 values) compared with haloperidol LAI. The main driver of the cost effectiveness of RLAI was the difference in Positive and Negative Syndrome Scale (PANSS) reduction between the two drugs, followed by the difference in adverse effects. The PSA showed that, in this setting, RLAI had a probability of 100% of being cost effective at a willingness-to-pay (WTP) threshold of €43 300 per QALY gained, compared with haloperidol LAI. The probability that RLAI combines additional effectiveness with cost savings compared with haloperidol LAI was estimated at 94%.
When analysing RLAI in the general schizophrenia population, it was predicted to generate 0.043 QALYs and save €239 per patient over 5 years compared with olanzapine. Compliance was the main driver of the cost effectiveness of RLAI in this scenario. In the PSA it was shown that RLAI had a probability of 78% of being cost effective at a WTP threshold of €43 300 per QALY gained, compared with olanzapine. The estimated probability that RLAI combines additional effectiveness with cost savings was 50% and the probability that RLAI is less effective and more costly than olanzapine was negligible (0.2%).
Conclusions
Treatment with RLAI is suggested to result in improved QALYs combined with cost savings compared with haloperidol LAI among the Swedish, high-risk non-compliant schizophrenia patient population. In the general schizophrenia population, RLAI also resulted in positive incremental QALYs and cost savings, when compared with olanzapine. However, the estimates used in the model for compliance and symptom reduction need further validation through naturalistic-based studies with reasonable follow-up to more definitely establish the real-life differences between RLAI and the comparators in the considered patient populations and to further reduce the uncertainty of these parameters.











Similar content being viewed by others
References
Lichtenstein P, Bjork C, Hultman CM, et al. Recurrence risks for schizophrenia in a Swedish national cohort. Psychol Med 2006 Oct; 36(10): 1417–25
Freedman R. Schizophrenia. N Engl J Med 2003 Oct 30; 349(18): 1738–49
Dental and Pharmaceutical Benefits Agency drug database [online]. Available from URL: http://www.tlv.se/beslut/sok/lakemedel/ [Accessed 2010 Jun 6]
Parellada E. Clinical experience and management considerations with long-acting risperidone. Curr Med Res Opin 2006 Feb; 22(2): 241–55
Keith SJ, Kane JM, Turner M, et al. Academic highlights: guidelines for the use of long-acting injectable atypical antipsychotics. J Clin Psychiatry 2004 Jan; 65(1): 120–31
Keith SJ, Pani L, Nick B, et al. Practical application of pharmacotherapy with long-acting risperidone for patients with schizophrenia. Psychiatr Serv 2004 Sep; 55(9): 997–1005
Heeg B, Buskens E, Knapp M, et al. Modelling the treated course of schizophrenia: development of a discrete event simulation model. Pharmaco-economics 2005; 23Suppl. 1: 17–33
Laux G, Heeg B, van Hout BA, et al. Costs and effects of long-acting risperidone compared with oral atypical and conventional depot formulations in Germany. Pharmaco-economics 2005; 23Suppl. 1: 49–61
Chue PS, Heeg B, Buskens E, et al. Modelling the impact of compliance on the costs and effects of long-acting risperidone in Canada. Pharmaco-economics 2005; 23Suppl. 1: 62–74
Heeg B, Buskens E, Botteman M, et al. The cost-effectiveness of atypicals in the UK. Value Health 2008 Dec; 11(7): 1007–21
National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in primary and secondary care (update). 2009 Mar 25 [online]. Available from URL: http://www.nice.org.uk/CG082 [Accessed 2010 Jun 6]
Socialstyrelsen (SoS). Swedish drug market [drug database; online]. Available from URL: http://www.socialstyrelsen.se/statistik/statistikefteramne/lakemedel [Accessed 2006 Dec 1]
Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005 Sep 22; 353(12): 1209–23
Schooler N, Rabinowitz J, Davidson M, et al. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry 2005 May; 162(5): 947–53
Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002 Jan 3; 346(1): 16–22
Leucht S, Barnes TR, Kissling W, et al. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry 2003 Jul; 160(7): 1209–22
Tollefson GD, Beasley Jr CM, Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry 1997 Apr; 154(4): 457–65
Hunt GE, Bergen J, Bashir M. Medication compliance and comorbid substance abuse in schizophrenia: impact on community survival 4 years after a relapse. Schizophr Res 2002 Apr 1; 54(3): 253–64
Hogarty GE. Depot neuroleptics: the relevance of psychosocial factors. A United States perspective. J Clin Psychiatry 1984 May; 45(5 Pt 2): 36–42
Rubio G, Martinez I, Ponce G, et al. Long-acting injectable risperidone compared with zuclopenthixol in the treatment of schizophrenia with substance abuse comorbidity. Can J Psychiatry 2006 Jul; 51(8): 531–9
Thieda P, Beard S, Richter A, et al. An economic review of compliance with medication therapy in the treatment of schizophrenia. Psychiatr Serv 2003 Apr; 54(4): 508–16
Dolder CR, Lacro JP, Dunn LB, et al. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry 2002 Jan; 159(1): 103–8
Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 2004 Apr; 161(4): 692–9
Fleischhacker WW, Oehl MA, Hummer M. Factors influencing compliance in schizophrenia patients. J Clin Psychiatry 2003; 64Suppl. 16: 10–3
Remington GJ, Adams ME. Depot neuroleptic therapy: clinical considerations. Can J Psychiatry 1995 Apr; 40(3 Suppl. 1): S5–11
Gerlach J. Depot neuroleptics in relapse prevention: advantages and disadvantages. Int Clin Psychopharmacol 1995 Jan; 9 Suppl. 5: 17–20
Keks NA, Ingham M, Khan A, et al. Long-acting injectable risperidone v. olanzapine tablets for schizophrenia or schizoaffective disorder: randomised, controlled, open-label study. Br J Psychiatry 2007 Aug; 191: 131–9
Kasper S, Lerman MN, McQuade RD, et al. Efficacy and safety of aripiprazole vs haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol 2003 Dec; 6(4): 325–37
Wirshing DA, Wirshing WC, Kysar L, et al. Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry 1999 Jun; 60(6): 358–63
Lieberman JA, Tollefson G, Tohen M, et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry 2003 Aug; 160(8): 1396–404
Koro CE, Fedder DO, L’italien GJ, et al. Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ 2002 Aug 3; 325(7358): 243
Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004 Mar; 161(3): 414–25
Socialstyrelsen Epidemiologiskt Centrum. Statistikdatabaser — diagnoser i slutenvård 2005 [database; online]. Available from URL: http://www.socialstyrelsen.se [Accessed in 2007 Jan 1]
WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index [database; online]. Available from URL: http://www.whocc.no/atc_ddd_index/ [Accessed 2007 Jan 1]
Läkemedelsförmansnämnden (LFN). Price database. 2007 [online]. Available from URL: http://www.tlv.se/beslut/sok/lakemedel/ [Accessed 2010 Jun 6]
Landstinget i Őstergötland. Priser och ersättningar för sydöstra sjukvardsregionen: Beslut I Regionsjukvardsnämden [online]. Available from URL: http://www.lio.se [Accessed 2010 Jul 5]
Socialstyrelsen och Statistiska centralbyran. Jämförelsetal för socialtjänsten ar 2005 [online]. Available from URL: http://www.socialstyrelsen.se/statistik/statistikefteramne/Sidor/jamforelsetalforsocialtjansten.aspx [Accessed 2010 Jul 5]
x-rates.com [online]. Available from URL: http://www.x-rates.com [Accessed 2007 Aug 7]
Statistics Sweden (Statistiska Centralbyrån). Inflation in Sweden 1831–2009 [online]. Available from URL: http://www.scb.se/Pages/TableAndChart33832.aspx [Accessed 2010 Jun 6]
Lenert LA, Sturley AP, Rapaport MH, et al. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schizophr Res 2004 Nov 1; 71(1): 155–65
Zhou H, Isaman DJ, Messinger S, et al. A computer simulation model of diabetes progression, quality of life, and cost. Diabetes Care 2005 Dec; 28(12): 2856–63
Landy J, Stein J, Brown MM, et al. Patient, community and clinician perceptions of the quality of life associated with diabetes mellitus. Med Sci Monit 2002 Aug; 8(8): CR543–8
Siddique RM, Markowitz J, Engelhart L. Impact of atypical antipsychotic drug sedation on quality of life [abstract no. NR765; plus poster]. American Psychiatric Association 2005 Annual Meeting; 2005 May 21; Atlanta (GA), 283–4
Pharmaceutical Benefits Board. General guidelines for economic evaluations from the Pharmaceutical Benefits Board [online]. Available from URL: http://tlv.se/Upload/English/ENG-lfnar-2003-2.pdf [Accessed 2010 Jun 6]
Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmaco-economics 2000 May; 17(5): 479–500
National Institute for Clinical Excellence. Guide to the methods of technology appraisal (no. n0515). London: NICE, 2004 Apr [online]. Available from URL: http://www.nice.org.uk/aboutnice/howwework/devnicetech/technologyappraisalprocessguides/guide_to_the_methods_of_technology_appraisal_reference_n0515.jsp [Accessed 2010 Jun 6]
Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 2003 Jun; 60(6): 553–64
Bagnall AM, Jones L, Ginnelly L, et al. A systematic review of atypical antipsychotic drugs in schizophrenia. Health Technol Assess 2003; 7(13): 1–193
Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ 2000 Dec 2; 321(7273): 1371–6
Emsley RA, Raniwalla J, Bailey PJ, et al. A comparison of the effects of quetiapine (’seroquel’) and haloperidol in schizophrenic patients with a history of and a demonstrated, partial response to conventional antipsychotic treatment. PRIZE Study Group. Int Clin Psychopharmacol 2000 May; 15(3): 121–31
Revicki DA, Genduso LA, Hamilton SH, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and other psychotic disorders: quality of life and clinical outcomes of a randomized clinical trial. Qual Life Res 1999 Aug; 8(5): 417–26
Lindenmayer JP, Czobor P, Volavka J, et al. Effects of atypical antipsychotics on the syndromal profile in treatment-resistant schizophrenia. J Clin Psychiatry 2004 Apr; 65(4): 551–6
Turner MS, Stewart DW. Review of the evidence for the long-term efficacy of atypical antipsychotic agents in the treatment of patients with schizophrenia and related psychoses. J Psychopharmacol 2006 Nov; 20(6 Suppl.): 20–37
Mahmoud RA, Engelhart LM, Janagap CC, et al. Risperidone versus conventional antipsychotics for schizophrenia and schizoaffective disorder: symptoms, quality of life and resource use under customary clinical care. Clin Drug Investig 2004; 24(5): 275–86
Kane JM, Meltzer HY, Carson Jr WH, et al. Aripiprazole for treatment-resistant schizophrenia: results of a multicenter, randomized, double-blind, comparison study versus perphenazine. J Clin Psychiatry 2007 Feb; 68(2): 213–23
Rabinowitz J, Lichtenberg P, Kaplan Z, et al. Rehospitalization rates of chronically ill schizophrenic patients discharged on a regimen of risperidone, olanzapine, or conventional antipsychotics. Am J Psychiatry 2001 Feb; 158(2): 266–9
Tunis SL, Faries DE, Nyhuis AW, et al. Cost-effectiveness of olanzapine as first-line treatment for schizophrenia: results from a randomized, open-label, 1-year trial. Value Health 2006 Mar; 9(2): 77–89
Damen J, Thuresson PO, Heeg B, et al. A pharmacoeconomic analysis of compliance gains on antipsychotic medications. Appl Health Econ Health Policy 2008; 6(4): 189–97
Niaz OS, Haddad PM. Thirty-five months experience of risperidone long-acting injection in a UK psychiatric service including a mirror-image analysis of in-patient care. Acta Psychiatr Scand 2007 Jul; 116(1): 36–46
Olivares JM, Rodriguez-Martinez A, Buron JA, et al. Cost-effectiveness analysis of switching antipsychotic medication to long-acting injectable risperidone in patients with schizophrenia: a 12- and 24-month follow-up from the e-STAR database in Spain. Appl Health Econ Health Policy 2008; 6(1): 41–53
Chang HC, Tang CH, Tsai SJ, et al. Long-acting injectable risperidone and hospital readmission: a mirror-image study using a national claim-based database in Taiwan. J Clin Psychiatry 2009 Jan; 70(1): 141
Acknowledgements
The authors thank Thomas Hanesjö (physician at the Norra Stockholms Psykiatri, SLSO), Maria Alnäs (physician at the Psykiatri Nordöst, SLSO) and Eva Lyssarides (physician at the Psykiatri Sydväst, SLSO) for their help in providing Swedish model input.
This study was sponsored by Janssen-Cilag Sweden. Mickael Löthgren is employed by Janssen-Cilag and was involved in the review of the manuscript. Marja Hensen and Bart Heeg are employed by, and Ben van Hout is a partner of, Pharmerit. Pharmerit holds consultancy contracts with Janssen-Cilag.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hensen, M., Heeg, B., Löthgren, M. et al. Cost effectiveness of long-acting risperidone in Sweden. Appl Health Econ Health Policy 8, 327–341 (2010). https://doi.org/10.2165/11536180-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11536180-000000000-00000