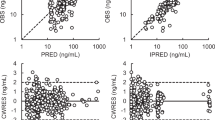
Abstract
Background and Objective
Gemcitabine (2′,2’-difluorodeoxycytidine) is an anticancer drug, which is effective against solid tumours, including non-small-cell lung cancer and pancreatic cancer. After gemcitabine is transported into cells by equilibrative and concentrative nucleoside transporters, it is phosphorylated by deoxycytidine kinase (DCK) and further phosphorylated to its active diphosphorylated and triphosphorylated forms. Gemcitabine is rapidly metabolized by cytidine deaminase (CDA) to an inactive metabolite, 2′,2′-difluorodeoxyuridine (dFdU), which is excreted into the urine. Toxicities of gemcitabine are generally mild, but unpredictable severe toxicities such as myelosuppression and interstitial pneumonia are occasionally encountered. The aim of this study was to determine the factors, including genetic polymorphisms of CDA, DCK and solute carrier family 29A1 (SLC29A1 [hENT1]), that alter the pharmacokinetics of gemcitabine in Japanese cancer patients.
Patients and Methods
250 Japanese cancer patients who received 30-minute intravenous infusions of gemcitabine at 800 or 1000mg/m2 in the period between September 2002 and July 2004 were recruited for this study. However, four patients were excluded from the final model built in this study because they showed bimodal concentration-time curves. Two patients who experienced gemcitabine-derived life-threatening toxicities in October 2006 and January 2008 were added to this analysis. One of these patients received 30-minute intravenous infusions of gemcitabine at 454 mg/m2 instead of the usual dose (1000 mg/m2).
Plasma concentrations of gemcitabine and dFdU were measured by high-performance liquid chromatography-photodiode array/mass spectrometry. In total, 1973 and 1975 plasma concentrations of gemcitabine and dFdU, respectively, were used to build population pharmacokinetic models using nonlinear mixed-effects modelling software (NONMEM® version V level 1.1).
Results and Discussion
Two-compartment models fitted well to plasma concentration-time curves for both gemcitabine and dFdU. Major contributing factors for gemcitabine clearance were genetic polymorphisms of CDA, including homozygous CDA*3 [208G>A (Ala70Thr)] (64% decrease), heterozygous *3 (17% decrease) and CDA -31delC (an approximate 7% increase per deletion), which has a strong association with CDA*2 [79A>C (Lys27Gln)], and coadministered S-1, an oral, multicomponent anti-cancer drug mixture consisting of tegafur, gimeracil and oteracil (an approximate 19% increase). The estimated contribution of homozygous CDA*3 to gemcitabine clearance provides an explanation for the life-threatening severe adverse reactions, including grade 4 neutropenia observed in three Japanese patients with homozygous CDA*3. Genetic polymorphisms of DCK and SLC29A1 (hENT1) had no significant correlation with gemcitabine pharmacokinetic parameters. Aging and increased serum creatinine levels correlated with decreased dFdU clearance.
Conclusion
A population pharmacokinetic model that included CDAgenotypes as a covariate for gemcitabine and dFdU in Japanese cancer patients was successfully constructed. The model confirms the clinical importance of the CDA*3 genotype.







Similar content being viewed by others
References
Noble S, Goa KL. Gemcitabine: a review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer. Drugs 1997; 54: 447–72
Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab 2004; 32: 63–84
Mackey JR, Mani RS, Selner M, et al. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res 1998; 58(19): 4349–57
Plunkett W, Huang P, Gandhi V. Preclinical characteristics of gemcitabine. Anticancer Drugs 1995; 6: S7–13
Heinemann V, Hertel LW, Grindey GB, et al. Comparison of the cellular pharmacokinetics and toxicity of 2′,2′-difluorodeoxycytidine and 1-beta-D-arabinofuranosylcytosine. Cancer Res 1988; 48(14): 4024–31
Kiani A, Kohne CH, Franz T, et al. Pharmacokinetics of gemcitabine in a patient with end-stage renal disease: effective clearance of its main metabolite by standard hemodialysis treatment. Cancer Chemother Pharmacol 2003; 51: 266–70
Aapro MS, Martin C, Hatty S. Gemcitabine: asafety review. Anticancer Drugs 1998; 9: 191–201
Gallelli L, Nardi M, Prantera T, et al. Retrospective analysis of adverse drug reactions induced by gemcitabine treatment in patients with non-small cell lung cancer. Pharmacol Res 2004; 49: 259–63
Locker GJ, Wenzel C, Schmidinger M, et al. Unexpected severe myelotoxicity of gemcitabine in pretreated breast cancer patients. Anticancer Drugs 2001; 12: 209–12
Sauer-Heilborn A, Kath R, Schneider CP, et al. Severe non-haematological toxicity after treatment with gemcitabine. J Cancer Res Clin Oncol 1999; 125: 637–40
Mercier C, Raynal C, Dahan L, et al. Toxic death case in a patient undergoing gemcitabine-based chemotherapy in relation with cytidine deaminase down-regulation. Pharmacogenet Genomics 2007; 17: 841–4
Sugiyama E, Kaniwa N, Kim SR, et al. Pharmacokinetics of gemcitabine in Japanese cancer patients: the impact of a cytidine deaminase polymorphism. J Clin Oncol 2007; 25: 32–42
Yonemori K, Ueno H, Okusaka T, et al. Severe drug toxicity associated with a single-nucleotide polymorphism of the cytidine deaminase gene in a Japanese cancer patient treated with gemcitabine plus cisplatin. Clin Cancer Res 2005; 11: 2620–4
Ueno H, Kaniwa N, Okusaka T, et al. Homozygous CDA*3 is a major cause of life-threatening toxicities in gemcitabine-treated Japanese cancer patients. Br J Cancer 2009; 100(6): 870–3
Kim SR, Saito Y, Maekawa K, et al. Twenty novel genetic variations and haplotype structures of the DCK gene encoding human deoxycytidine kinase (dCK). Drug Metab Pharmacokinet 2008; 23(5): 379–84
Kim SR, Saito Y, Maekawa K, et al. Thirty novel genetic variations in the SLC29A1 gene encoding human equilibrative nucleoside transporter 1 (hENT1). Drug Metab Pharmacokinet 2006; 21: 248–56
De Pas T, De Braud F, Danesi R, et al. Phase I and pharmacologic study of weekly gemcitabine and paclitaxel in chemo-naive patients with advanced non-small-cell lung cancer. Ann Oncol 2000; 11: 821–7
Hooker AC, Staats CE, Karlsson MO. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 2007; 24: 2187–97
Shi JY, Shi ZZ, Zhang SJ, et al. Association between single nucleotide poly-morphisms in deoxycytidine kinase and treatment response among acute myeloid leukaemia patients. Pharmacogenetics 2004; 14(11): 759–68
Lamba JK, Crews K, Pounds S, et al. Pharmacogenetics of deoxycytidine kinase: identification and characterization of novel genetic variants. J Pharmacol Exp Ther 2007; 323(3): 935–45
Jiang X, Galettis P, Links M, et al. Population pharmacokinetics of gemcitabine and its metabolite in patients with cancer: effect of oxaliplatin and infusion rate. Br J Clin Pharmacol 2008; 65: 326–33
Tham LS, Wang LZ, Soo RA, et al. Does saturable formation of gemcitabine triphosphate occur in patients? Cancer Chemother Pharmacol 2008; 63: 55–64
Giovannetti E, Laan AC, Vasile E, et al. Correlation between cytidine deaminase genotype and gemcitabine deamination in blood samples. Nucleosides Nucleotides Nucleic Acids 2008; 27: 720–5
Tibaldi C, Giovannetti E, Vasile E, et al. Correlation of CDA, ERCC1, and XPD polymorphisms with response and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res 2008; 14: 1797–803
Tsujie M, Nakamori S, Nakahira S, et al. Human equilibrative nucleoside transporter 1, as a predictor of 5-fluorouracil resistance in human pancreatic cancer. Anticancer Res 2007; 27: 2241–9
Nakahira S, Nakamori S, Tsujie M, et al. Pretreatment with S-1, an oral derivative of 5-fluorouracil, enhances gemcitabine effects in pancreatic cancer xenografts. Anticancer Res 2008; 28: 179–86
Abbruzzese JL, Grunewald R, Weeks EA, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol 1997; 9: 491–8
Acknowledgements
We thank Eli Lilly Japan KK (Kobe, Japan) for kindly providing gemcitabine and dFdU for analytical standards. We thank the patients for participating in this study and Ms Emi Toshiro, Ms Tomoko Chujo, Ms Emiko Usami, Ms Tomoko Matsumura and Ms Mamiko Shimada for assistance in sample collection and processing. We also thank Ms Chie Sudo for secretarial assistance. This study was supported in part by the Program for the Promotion of Fundamental Studies in Health Sciences at the National Institute of Biomedical Innovation [NiBio] (Osaka, Japan) and by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare (Tokyo, Japan).
Dr Okusaka reported receiving honoraria from Eli Lilly. The other authors reported no financial disclosures and have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugiyama, E., Kaniwa, N., Kim, SR. et al. Population Pharmacokinetics of Gemcitabine and Its Metabolite in Japanese Cancer Patients. Clin Pharmacokinet 49, 549–558 (2010). https://doi.org/10.2165/11532970-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11532970-000000000-00000