Abstract
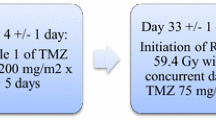
Carmustine (BCNU: N,N’-bis[2-chloroethyl]-N-nitrosourea) wafers are a local chemotherapeutic agent for the treatment of malignant glioma. They avoid the problems of high toxicity and short half-life associated with systemic delivery, and can bridge the traditional ‘treatment gap’ between surgery and subsequent conventional chemo- or radiotherapy. Clinical trials have demonstrated significant improvements in survival and quality of life for patients after complete tumour resection and BCNU wafer implantation. In practice, clinicians may use BCNU wafers in conjunction with other radio- and chemotherapies, in order to maximize the chance of a beneficial patient outcome. The purpose of these case reports is to exemplify how four experienced European clinicians employ BCNU wafers for the management of malignant glioma, and to illustrate how BCNU wafers can be effectively incorporated into treatment regimens. Four patients are described in whom BCNU wafers were implanted during the course of treatment for glioblastoma multiforme, the most severe and common type of malignant glioma. These include three patients with recurrent disease, and a single patient with a newly diagnosed tumour. All four patients received additional radio- and chemotherapy as appropriate. Treatment was well tolerated and patient survival from diagnosis ranged from 56 to 132 weeks. This compared favourably with the survival of approximately 58 weeks seen in the recent EORTC-NCIC clinical trial of combined radiotherapy with concomitant and adjuvant temozolomide. BCNU wafers are an effective means of increasing survival and quality of life in patients diagnosed with malignant glioma, and are a valuable addition to the overall multimodal treatment strategy for these tumours.




Similar content being viewed by others
References
Boyle P, Levin B, editors. World cancer report 2008. Lyon: International Agency for Research on Cancer, 2008
Rachet B, Mitry E, Quinn MJ, et al. Survival from brain tumours in England and Wales up to 2001. Br J Cancer 2008 Sep 23; 99Suppl. 1: S98–101
UK brain and central nervous system cancer incidence statistics [online]. Available from URL: http://info.cancerresearchuk.org/cancerstats/types/brain/incidence/ [Accessed 2009 Jan 4]
Bauchet L, Rigau V, Mathieu-Daude H, et al. French brain tumor data bank: methodology and first results on 10,000 cases. J Neurooncol 2007 Sep; 84(2): 189–99
Ekman M, Westphal M. Cost of brain tumour in Europe. Eur J Neurol 2005 Jun; 12Suppl. 1: 45–9
Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol 2005 Jan; 109(1): 93–108
Stupp R, Roila F. Malignant glioma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008 May; 19Suppl. 2: ii83–5
Sant M, Aareleid T, Berrino F, et al. EUROCARE-3: survival of cancer patients diagnosed 1990-9 — results and commentary. Ann Oncol 2003; 14Suppl. 5: v61–118
Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009 May; 10(5): 459–66
Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 2005 Jun; 64(6): 479–89
Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology 1980 Sep; 30(9): 907–11
Kochi M, Ushio Y. High-dose chemotherapy with autologous hematopoietic stem-cell rescue for patients with malignant brain tumors. Crit Rev Neurosurg 1999 Sep 24; 9(5): 295–302
Petersdorf SH, Livingston RB. High dose chemotherapy for the treatment of malignant brain tumors. J Neurooncol 1994; 20(2): 155–63
Zucchetti M, Boiardi A, Silvani A, et al. Distribution of daunorubicin and daunorubicinol in human glioma tumors after administration of liposomal daunorubicin. Cancer Chemother Pharmacol 1999; 44(2): 173–6
Gumerlock MK, Belshe BD, Madsen R, et al. Osmotic blood-brain barrier disruption and chemotherapy in the treatment of high grade malignant glioma: patient series and literature review. J Neurooncol 1992 Jan; 12(1): 33–46
Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol 2000 Apr; 20(2): 217–30
Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep 1983 Feb; 67(2): 121–32
Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 1980 Dec 4; 303(23): 1323–9
Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 2003 Apr; 5(2): 79–88
Lin SH, Kleinberg LR. Carmustine wafers: localized delivery of chemotherapeutic agents in CNS malignancies. Expert Rev Anticancer Ther 2008 Mar; 8(3): 343–59
Grossman SA, Reinhard C, Colvin OM, et al. The intracerebral distribution of BCNU delivered by surgically implanted biodegradable polymers. J Neurosurg 1992 Apr; 76(4): 640–7
Dang W, Daviau T, Brem H. Morphological characterization of polyanhydride biodegradable implant Gliadel during in vitro and in vivo erosion using scanning electron microscopy. Pharm Res 1996 May; 13(5): 683–91
Fung LK, Ewend MG, Sills A, et al. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclo-phosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res 1998 Feb 15; 58(4): 672–84
Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet 2002; 41(6): 403–19
Sampath P, Brem H. Implantable slow-release chemotherapeutic polymers for the treatment of malignant brain tumors. Cancer Control 1998 Mar; 5(2): 130–7
Gururangan S, Cokgor L, Rich JN, et al. Phase I study of Gliadel wafers plus temozolomide in adults with recurrent supratentorial high-grade gliomas. Neuro Oncol 2001 Oct; 3(4): 246–50
Welcome healthcare professionals [online]. Available from URL: http://www.gliadel.com/hcp/default.aspx [Accessed 2009 Nov 4]
Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 1995 Apr 22; 345(8956): 1008–12
Westphal M, Ram Z, Riddle V, et al. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 2006 Mar; 148(3): 269–75; discussion 75
Do V, Gebski V, Barton MB. The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol 2000 Nov; 57(2): 131–6
Gliadel 7.7 mg implant summary of product characteristics [online]. Available from URL: http://emc.medicines.org.uk/emc/assets/c/html/DisplayDoc.asp?DocumentID = 13913 [Accessed 2009 Jan 6]
Temodal: summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu/humandocs/Humans/EPAR/temodal/temodal.htm [Accessed 2009 Nov 4]
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005 Mar 10; 352(10): 987–96
Ashby LS, Ryken TC. Management of malignant glioma: steady progress with multimodal approaches. Neurosurg Focus 2006; 20(4): E3
La Rocca RV, Mehdorn HM. Localized BCNU chemotherapy and the multimodal management of malignant glioma. Curr Med Res Opin 2009; 25(1): 149–60
Spiegel BM, Esrailian E, Laine L, et al. Clinical impact of adjuvant chemotherapy in glioblastoma multiforme: a meta-analysis. CNS Drugs 2007; 21(9): 775–87
McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg 2009 Mar; 110(3): 583–8
Pan E, Mitchell SB, Tsai JS. A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neurooncol 2008 Jul; 88(3): 353–7
Carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma [online]. Available from URL: http://www.nice.org.uk/nicemedia/pdf/TA121quickrefguide.pdf [Accessed 2009 Jan 6]
Engelhard HH. Tumor bed cyst formation after BCNU wafer implantation: report of two cases. Surg Neurol 2000 Mar; 53(3): 220–4
McGirt MJ, Villavicencio AT, Bulsara KR, et al. Management of tumor bed cysts after chemotherapeutic wafer implantation: report of four cases. J Neurosurg 2002 May; 96(5): 941–5
Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 2000 Nov 9; 343(19): 1350–4
Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol 2002 May 1; 20(9): 2388–99
Pegg AE. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res 1990 Oct 1; 50(19): 6119–29
Mitchell RB, Dolan ME. Effect of temozolomide and dacarbazine on O6-alkylguanine-DNA alkyltransferase activity and sensitivity of human tumor cells and xenografts to 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Chemother Pharmacol 1993; 32(1): 59–63
Plowman J, Waud WR, Koutsoukos AD, et al. Preclinical antitumor activity of temozolomide in mice: efficacy against human brain tumor xenografts and synergism with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res 1994 Jul 15; 54(14): 3793–9
Rhines LD, Sampath P, Dolan ME, et al. O6-benzylguanine potentiates the antitumor effect of locally delivered carmustine against an intracranial rat glioma. Cancer Res 2000 Nov 15; 60(22): 6307–10
Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 2008 Sep 1; 26(25): 4189–99
Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst 2007 Nov 7; 99(21): 1583–93
Sabel M, Giese A. Safety profile of carmustine wafers in malignant glioma: a review of controlled trials and a decade of clinical experience. Curr Med Res Opin 2008 Oct 20; 24(11): 3239–57
Evans D. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare interventions. J Clin Nurs 2003 Jan; 12(1): 77–84
Acknowledgements
Lutz Dörner is a consultant and clinical trial investigator for Archimedes Pharma. Evelyne Emery has received financial support from Archimedes Pharma to attend scientific meetings. Oliver Heese has been a consultant and speaker for Archimedes Pharma, Codman and Essex Pharma GmbH. H. Maximilian Mehdorn has served as a speaker and on advisory boards for Archimedes Pharma. Philippe Menei is a consultant for Biocompatibles International plc and has acted as a speaker for Archimedes Pharma and Schering-Plough. Anne Balossier and Jagmohan Singh have no conflicts of interest that are directly relevant to the content of this article. No funding was received by the authors regarding this manuscript. All authors had complete access to the data, reviewed each manuscript draft and approved the final manuscript. Editorial support was provided by Fishawack Communications Ltd and funded by Archimedes Pharma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balossier, A., Dörner, L., Emery, E. et al. Incorporating BCNU Wafers into Malignant Glioma Treatment. Clin. Drug Investig. 30, 195–204 (2010). https://doi.org/10.2165/11532900-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11532900-000000000-00000