Abstract
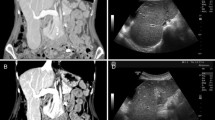
We report a case of acute-onset, long-lasting cholestasis induced by atorvastatin. This antihyperlipidaemic drug was taken for 40 days by a 72-year-old male as a treatment for his mixed dyslipidaemia. At that point, the patient presented with asthenia, nausea, painless icterus, acholic stools and hyperchromic urine with biochemical analyses showing a dramatic increase in bilirubin (total bilirubin 22mg/dL; direct bilirubin 21 mg/dL) and alkaline phosphatase (up to 4-fold over the normal level) with less marked increases in transaminases. Liver histology showed a pattern of cholestasis with evident signs of cholangiolitis and damage of the interlobular bile ducts. Serum transaminase and bilirubin levels returned to normal within 5 months after atorvastatin withdrawal while alkaline phosphatase normalized after only 8 months. Scores on both the Maria and Victorino clinical scale for the diagnosis of drug-induced hepatitis and the Naranjo Adverse Drug Reaction Probability Scale indicated that atorvastatin was the probable cause of prolonged cholestasis in this patient. This is a rare case of cholestasis probably caused by atorvastatin and unusually characterized by bile duct damage.


Similar content being viewed by others
References
Yusuf S, Lonn E, Bosch J. Lipid lowering for primary prevention. Lancet 2009; 373: 1152–5
Onusko E. Statins and elevated liver tests: what’s the fuss? J Fam Pract 2008; 57(7): 449–52
Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf 2007; 6(6): 673–84
Wierzbicki AS. Atorvastatin. Expert Opin Pharmacother 2001; 2(5): 819–30
Recto 2nd CS, Acosta S, Dobs A. Comparison of the efficacy and tolerability of simvastatin and atorvastatin in the treatment of hypercholesterolemia. Clin Cardiol 2000; 23(9): 682–8
Clarke AT, Mills PR. Atorvastatin associated liver disease. Dig Liver Dis 2006; 38(10): 772–7
Abourjaily HM, Alsheikh-Ali AA, Karas RH. Comparison of the frequency of adverse events in patients treated with atorvastatin or simvastatin. Am J Cardiol 2003 Apr 15; 91(8): 999–1002
Malhotra HS, Goa KL. Atorvastatin: an updated review of its pharmacological properties and use in dyslipidaemia. Drugs 2001; 61(12): 1835–81
Yee HS, Fong NT. Atorvastatin in the treatment of primary hypercholesterolemia and mixed dyslipidemias. Ann Pharmacother 1998; 32(10): 1030–43
Black DM, Bakker-Arkema RG, Nawrocki JW. An overview of the clinical safety profile of atorvastatin (Lipitor), a new HMG-CoA reductase inhibitor. Arch Intern Med 1998 Mar 23; 158(6): 577–84
Newman CB, Palmer G, Silbershatz H, et al. Safety of atorvastatin derived from analysis of 44 completed trials in 9,416 patients. Am J Cardiol 2003 Sep 15; 92(6): 670–6
Rahier JF, Rahier J, Leclercq I, et al. Severe acute chole-static hepatitis with prolonged cholestasis and bile-duct injury following atorvastatin therapy: a case report. Acta Gastroenterol Belg 2008; 71(3): 318–20
Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. Am J Cardiol 2006 Apr 17; 97(8A): 44C–51C
Suzuki A, Yuen N, Walsh J, et al. Co-medications modulating liver injury and repair influence clinical outcome of acetaminophen-associated liver injury. Clin Gastroenterol Hepatol 2009; 7(8): 882–8
Arca M. Atorvastatin: a safety and tolerability profile. Drugs 2007; 67Suppl. 1: 63–9
Stojakovic T, Putz-Bankuti C, Fauler G, et al. Atorvastatin in patients with primary biliary cirrhosis and incomplete biochemical response to ursodeoxycholic acid. Hepatology 2007; 46(3): 776–84
Andrade RJ, Lucena MI, Kaplowitz N, et al. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology 2006; 44(6): 1581–8
Jiménez-Alonso J, Osorio JM, Gutiérrez-Cabello F, et al. Atorvastatin-induced cholestatic hepatitis in a young woman with systemic lupus erythematosus. Arch Intern Med 1999; 159(15): 1811–2
De Castro ML, Hermo JA, Baz A, et al. Acute cholestatic hepatitis after atorvastatin reintroduction. Gastroenterol Hepatol 2006; 29(1): 21–4
Ridruejo E, Mandó OG. Acute cholestatic hepatitis after reinitiating treatment with atorvastatin. J Hepatol 2002; 37(1): 165–6
Armitage J. The safety of statins in clinical practice. Lancet 2007 Nov 24; 370(9601): 1781–90
Lee WM. Drug-induced hepatotoxicity. N Engl J Med 2003 Jul 31; 349(5): 474–85
Bénichou C. Criteria of drug-induced liver disorders: report of an international consensus meeting. J Hepatol 1990; 11(2): 272–6
Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology 1997; 26(3): 664–9
Naranjo CA, Busto U, Sellers EM, et al. A reliable method for estimating the probability of adverse drug reactions. Clin Pharmac Ther 1981; 30: 239–45
Acknowledgements
No sources of funding were used to assist in the preparation of this case report. The authors have no conflicts of interest that are directly relevant to the content of this report. The authors thank Tracie Dornbusch for editing the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Merli, M., Bragazzi, M.C., Giubilo, F. et al. Atorvastatin-Induced Prolonged Cholestasis with Bile Duct Damage. Clin. Drug Investig. 30, 205–209 (2010). https://doi.org/10.2165/11531660-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11531660-000000000-00000