Abstract
Background and Objectives: Transdermal patches provide non-invasive, continuous drug delivery, and offer significant potential advantages over oral treatments. With all transdermal treatments a proportion of patients will experience some form of skin reaction. The rivastigmine patch has been approved for the treatment of mild-to-moderate Alzheimer’s disease (AD) since July 2007 in the US. The aim of the component of the trial reported here was to evaluate the skin tolerability of the rivastigmine transdermal patch in patients with mild-to-moderate AD.
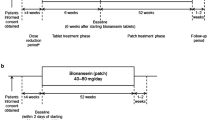
Methods: The pivotal IDEAL trial was a 24-week, randomized, double-blind, placebo-controlled, multicentre trial of the efficacy and tolerability of the rivastigmine transdermal patch in 1195 patients with mild-to-moderate AD. This was followed by a 28-week open-label extension. Although not prospectively defined as a secondary assessment, during both phases of the study the condition of the patients’ skin at the application site was evaluated. These data are reviewed in this article.
Results: During the 24-week, double-blind phase of the study, 89.6% of patients in the target 9.5 mg/24 h patch treatment group had recorded ‘no, slight or mild’ signs or symptoms for their most severe application-site reaction. Erythema and pruritus were the most commonly reported reactions. No patient in any patch treatment group experienced a skin reaction that was reported as a serious adverse event. In the 9.5 mg/24 h treatment group, 2.4% of patients discontinued treatment due to an application-site reaction. During the 28-week open-label extension, the skin tolerability profile was similar to that seen in the double-blind phase. Overall, 3.7% of patients discontinued treatment due to application-site skin reactions. There was no indication that the severity of the skin reactions increased over time.
Conclusion: Overall, the data support a favourable skin tolerability profile for the rivastigmine transdermal patch, and provide reassurance that the benefits of rivastigmine patch therapy for patients with AD are not confounded by significant skin irritation problems. Nevertheless, care should be taken to follow manufacturer’s advice about patch application, such as daily rotation of the application site, to minimize the risk of skin reactions.





Similar content being viewed by others
References
Nitti VW, Sanders S, Staskin DR, et al. Transdermal delivery of drugs for urologic applications: basic principles and applications. Urology 2006; 67: 657–64
Petersen TA. Transdermal drug formulations and process development. Pharmaceutical Technology 2003; 27(6 Suppl.): 18–21
Priano L, Gasco MR, Mauro A. Transdermal treatment options for neurological disorders: impact on the elderly. Drugs Aging 2006; 23: 357–75
Small G, Dubois B. A review of compliance to treatment in Alzheimer’s disease: potential benefits of a transdermal patch. Curr Med Res Opin 2007; 23: 2705–13
Wolf R, Tüzün B, Tüzün Y. Adverse skin reactions to the nicotine transdermal system. Clin Dermatol 1998; 16: 617–23
Widman TJ, Oostman H, Storrs FJ. Allergic contact dermatitis from medical adhesive bandages in patients who report having a reaction to medical bandages. Dermatitis 2008; 19: 32–7
Prisant LM. Transdermal clonidine skin reactions. J Clin Hypertens (Greenwich) 2002; 4: 138–8
Levin C, Maibach HI. Transdermal drug delivery system: an overview. In: Marzulli FN, Maibach HI, editors. Marzulli and Maibach’s dermatotoxicology. 7th ed. Boca Raton (FL): CRC Press, 2008: 101–6
Upadhye MR, Maibach HI. Influence of area of application of allergen on sensitization in contact dermatitis. Contact Dermatitis 1992; 27: 281–6
Weltfriend S, Maibach HI. Irritant dermatitis: clinical heterogeneity and contributing factors. In: Marzulli FN, Maibach HI, editors. Marzulli and Maibach’s dermatotoxicology. 7th ed. Boca Raton (FL): CRC Press, 2008: 125–38
Hurkmans JF, Boddé HE, Van Driel LM, et al. Skin irritation caused by transdermal drug delivery systems during long-term (5 days) application. Br J Dermatol 1985; 112: 461–7
Winblad B, Machado JC. Use of rivastigmine transdermal patch in the treatment of Alzheimer’s disease. Exp Opin Drug Deliv 2008; 5: 1–10
Winblad B, Cummings J, Andreasen N, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease: rivastigmine patch versus capsule. Int J Geriatr Psychiatry 2007; 22: 456–67
Grossberg G, Sadowsky C, Förstl H, et al. Safety and tolerability of the rivastigmine patch: results of a 28-week open-label extension. Alzheimer Dis Assoc Disord 2009; 23: 158–64
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). 4th ed. Washington, DC: American Psychiatric Association, 1994
McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 1984; 34: 939–44
US Food and Drug Administration. What is a serious adverse event? [online]. Available from URL: http://www.fda.gov/Safety/MedWatch/HowToReport/ucm053087.htm [Accessed 2009 Sep 22]
Data on file. Novartis Pharmaceuticals Corporation, East Hanover (NJ): 2007 Jul
Rosenstein LD. Differential diagnosis of the major progressive dementias and depression in middle and late adulthood: a summary of the literature of the early 1990s. Neuropsychol Rev 1998; 8: 109–67
Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol 2005; 11: 221–35
Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol 2006; 12: 145–54
Fisher AA. Dermatitis due to transdermal therapeutic systems. Cutis 1984; 34: 526–7, 530-531
Bodkin JA, Amsterdam JD. Transdermal selegiline in major depression: a double-blind, placebo-controlled, parallel-group study in outpatients. Am J Psychiatry 2002; 159: 1869–75
LeWitt PA, Lyons KE, Pahwa R, et al. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER study. Neurology 2007; 68: 1262–7
Murphy M, Carmichael AJ. Transdermal drug delivery systems and skin sensitivity reactions: incidence and management. Am J Clin Dermatol 2000; 1: 361–8
Marzulli FN, Maibach HI. Allergic contact dermatitis. In: Marzulli FN, Maibach HI, editors. Marzulli and Maibach’s dermatotoxicology. 7th ed. Boca Raton (FL): CRC Press, 2008: 155–8
FDA approved Exelon® Patch label [package insert]. East Hanover (NJ): Novartis Pharmaceuticals Corporation, 2007 July
Held E, Agner T. Effect of moisturizers on skin susceptibility to irritants. Acta Derm Venereol 2001; 81: 104–7
Held E, Lund H, Agner T. Effect of different moisturizers on SLS-irritated human skin. Contact Dermatitis 2001; 44: 229–34
Zhai H, Anigbogu AN, Maibach HI. Irritant and allergic contact dermatitis treatment. In: Marzulli FN, Maibach HI, editors. Marzulli and Maibach’s dermatotoxicology. 7th ed. Boca Raton (FL): CRC Press, 2008: 69–96
Amkraut AA, Jordan WP, Taskovich L. Effect of coadministration of corticosteroids on the development of contact sensitization. J Am Acad Dermatol 1996; 35: 27–31
Acknowledgements
This article is based on data derived from a double-blind, randomized global study of the rivastigmine transdermal patch; the site investigators are acknowledged for the collection of data. Novartis supported this study and the development and manufacture of the rivastigmine patch. All authors contributed to the content, critical review and approval of the manuscript. Jeffrey Cummings has provided expert consultation services to and received honoraria from Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. Martin Farlow has provided expert consultation services to and received speaker honoraria and research funding from Novartis Pharmaceuticals Corporation. Xiangyi Meng, Sibel Tekin and Jason Olin are employees of and own stock ownership options in Novartis Pharmaceuticals Corporation. Prof. Howard I. Maibach, University of California, San Francisco, CA, USA, acted as an expert clinical consultant for this manuscript and provided important intellectual input. Stuart Wakelin from Alpha-Plus Medical Communications Ltd (UK) provided editorial and administrative support in the production of this manuscript, which was sponsored by Novartis Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cummings, J.L., Farlow, M.R., Meng, X. et al. Rivastigmine Transdermal Patch Skin Tolerability. Clin. Drug Investig. 30, 41–49 (2010). https://doi.org/10.2165/11531270-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11531270-000000000-00000