Abstract
Ingenol mebutate is the main active constituent of sap from the plant Euphorbia peplus, which has traditionally been used as a home remedy for various skin conditions. Ingenol mebutate gel is approved in the US, EU, Australia and Brazil for the topical treatment of actinic keratosis.
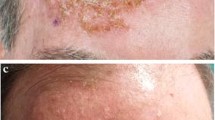
A short course of field-directed therapy with topical ingenol mebutate gel was effective in the treatment of actinic keratoses on the face or scalp (ingenol mebutate gel 0.015% once daily for 3 consecutive days) and on the trunk or extremities (ingenol mebutate gel 0.05% once daily for 2 consecutive days), according to the results of four randomized, double-blind, vehicle-controlled, multicentre studies.
Significantly higher complete clearance rates (primary endpoint) and partial clearance rates were seen at day 57 in patients receiving ingenol mebutate gel than in those receiving vehicle gel. Treatment with ingenol mebutate gel was generally associated with sustained clearance of actinic keratoses in the longer term.
Topical ingenol mebutate gel was generally well tolerated in the treatment of patients with actinic keratoses on the face or scalp and on the trunk or extremities. Application-site conditions were the most commonly occurring adverse events.
Similar content being viewed by others
References
de Berker D, McGregor JM, Hughes BR, et al. Guidelines for the management of actinic keratoses. Br J Dermatol 2007 Feb; 156(2): 222–30
Del Rosso JQ. Current regimens and guideline implications for the treatment of actinic keratosis: proceedings of a clinical roundtable at the 2011 Winter Clinical Dermatology Conference. Cutis 2011 Jul; 88 Suppl. 1: 1–8
Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol 2009 Jun; 60(6): 934–43
Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion-specific immune response. J Am Acad Dermatol 2012 Mar; 66(3): 486–93
Barnaby JWJ, Styles AR, Cockerell CJ. Actinic keratoses: differential diagnosis and treatment. Drugs Aging 1997 Sep; 11(3): 186–205
Ramsay JR, Suhrbier A, Aylward JH, et al. The sap from Euphorbia peplus is effective against human nonmelanoma skin cancers. Br J Dermatol 2011 Mar; 164(3): 633–6
Rizk AM, Hammouda FM, El-Missiry MM, et al. Biologically active diterpene esters from Euphorbia peplus. Phytochem 1985; 24(7): 1605–6
LEO Pharma, Inc. Picato® (ingenol mebutate) gel, 0.015% and 0.05% for topical use: US prescribing information [online] Available from URL: http://www.leo-pharma.us/Files/Billeder/LEO_local_images/LEO-Pharma.US/Releases/Picato%20PI.pdf [Accessed 2012 Jun 28]
European Medicines Agency. Picato (ingenol mebutate) gel: EU summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002275/WC500135327.pdf [Accessed 2012 Dec 3]
Stahlhut M, Chahal H, Lord J, et al. Ingenol mebutate initiates multiple specific cell death pathways in human cancer cells [abstract no. 5517]. J Am Acad Dermatol 2012 Apr; 66 (4 Suppl. 1): AB152
Zibert JR, Eriksson AH, Grue-Sørensen G, et al. Ingenol mebutate penetrates reconstructed human skin in a gradient-dependent manner and clears subclinical skin cancer [abstract no. 5501]. J Am Acad Dermatol Apr; 66 (4 Suppl. 1): AB152
Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol 2006 Dec 1; 177(11): 8123–32
Ogbourne S, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res 2004 Apr 15; 64(8): 2833–9
Hampson P, Kavanagh D, Smith E, et al. The anti-tumor agent, ingenol-3-angelate (PEP005), promotes the recruitment of cytotoxic neutrophils by activation of vascular endothelial cells in a PKC-δ dependent manner. Cancer Immunol Immunother 2012 Aug; 57(8): 1241–51
Kedei N, Lundberg DJ, Toth A, et al. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res 2004 May 1; 64(9): 3243–55
Ersvaer E, Kittang AO, Hampson P, et al. The protein kinase C agonist PEP005 (ingenol 3-angelate) in the treatment of human cancer: a balance between efficacy and toxicity. Toxins 2010 Jan; 2(1): 174–94
Li L, Shukla S, Lee A, et al. The skin cancer chemotherapeutic agent ingenol-3-angelate (PEP005) is a substrate for the epidermal multidrug transporter (ABCB1) and targets tumor vasculature. Cancer Res 2010 Jun 1; 70(11): 4509–19
Cozzi S-J, Ogbourne SM, James C, et al. Ingenol mebutate field-directed treatment of UVB-damaged skin reduces lesion formation and removes mutant p53 patches. J Invest Dermatol 2012 Apr; 132(4): 1263–71
Peplin. A study to evaluate the pharmacokinetics of PEP005 (ingenol mebutate) gel, 0.05%, when applied in a maximal use setting to the dorsal aspect of the forearm in patients with actinic keratosis [ClinicalTrials.gov identifier NCT00852137]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials/gov [Accessed 2012 Jun 28]
Siller G, Gebauer K, Welburn P, et al. PEP005 (ingenol mebutate) gel, a novel agent for the treatment of actinic keratosis: results of a randomized, double-blind, vehicle-controlled, multicentre, phase IIa study. Australas J Dermatol 2009 Feb; 50(1): 16–22
Spencer J. Multicenter, randomized, double-blind, vehicle-controlled, dose-ranging study to evaluate the efficacy and safety of PEP005 (ingenol mebutate) gel 0.005%, 0.01%, and 0.015% when used to treat actinic keratoses on the head [abstract no. P2913]. J Am Acad Dermatol 2010 Mar; 62 (3 Suppl. 1): AB105
Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med 2012 Mar 15; 366(11): 1010–9
Berman B, Marmur E, Larsson T, et al. Three-day topical treatment with ingenol mebutate gel 0.015% for actinic keratoses on the face and scalp: analysis of data pooled from two trials [poster no. 5623]. 70th Annual Meeting of the American Academy of Dermatology; 2012 Mar 16–20; San Diego (CA)
Anderson LL, Schmieder GJ, Xu Z, et al. Two-day topical treatment with ingenol mebutate gel 0.05% for actinic keratoses on the trunk and extremities: analysis of data pooled from two trials [poster no. 5640]. 70th Annual Meeting of the American Academy of Dermatology; 2012 Mar 16–20; San Diego (CA)
Stein Gold L, Melgaard A, Larsson T. Long-term follow-up studies of ingenol mebutate gel for the treatment of actinic keratosis [abstract no. 5620]. J Am Acad Dermatol 2012 Apr; 66 (4 Suppl. 1): AB154. Plus poster presented at the 70th Annual Meeting of the American Academy of Dermatology; 2012 Mar 16–20; San Diego (CA)
Peplin. A multi-center study to evaluate the safety and efficacy of PEP005 (ingenol mebutate) gel, when used to treat actinic keratoses on non-head locations (trunk and extremities) [ClinicalTrials.gov identifier NCT00917306]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials/gov [Accessed 2012 Jul 16]
Lebwohl M, Swanson N, Kobayashi K, et al. Local skin responses associated with ingenol mebutate gel for the treatment of actinic keratosis: two analyses of pooled data [poster no. 4997]. 70th Annual Meeting of the American Academy of Dermatology; 2012 Mar 16–20; San Diego (CA)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keating, G.M. Ingenol Mebutate Gel 0.015% and 0.05%. Drugs 72, 2397–2405 (2012). https://doi.org/10.2165/11470090-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11470090-000000000-00000