Abstract
Background
Duloxetine is indicated for patients with a variety of conditions, and some of these patients may have mild to moderate degrees of renal impairment. Renal impairment may affect the pharmacokinetics of a drug by causing changes in absorption, distribution, protein binding, renal excretion or nonrenal clearance. As duloxetine is highly bound to plasma proteins and its metabolites are renally excreted, it is prudent to evaluate the effect of renal insufficiency on exposure to duloxetine and its metabolites in the systemic circulation.
Objective
The aim of this study was to evaluate the effects of varying degrees of renal impairment on duloxetine pharmacokinetics in a single-dose phase I study and using pooled steady-state pharmacokinetic data from phase II/III trials.
Methods
In the phase I study, a single oral dose of duloxetine 60 mg was given to 12 subjects with end-stage renal disease (ESRD) and 12 matched healthy control subjects. In the phase II/III trials (n = 463 patients), duloxetine 20–60 mg was given as once- or twice-daily doses. Duloxetine and metabolite concentrations in plasma were determined using liquid chromatography with tandem mass spectrometry. Noncompartmental methods (phase I: duloxetine and its metabolites) and population modelling methods (phase II/III: duloxetine) were used to analyse the pharmacokinetic data.
Results
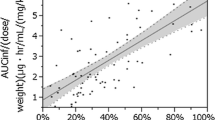
The maximum plasma concentration (Cmax) and the area under the plasma concentration-time curve (AUC) of duloxetine were ∼2-fold higher in subjects with ESRD than in healthy subjects, which appeared to reflect an increase in oral bioavailability. The Cmax and AUC of two major inactive conjugated metabolites were as much as 2- and 9-fold higher, respectively, reflecting reduced renal clearance of these metabolites. Population pharmacokinetic results indicated that mild or moderate renal impairment, assessed by creatinine clearance (CLCR) calculated according to the Cockcroft-Gault formula, did not have a statistically significant effect on pharmacokinetic parameters of duloxetine. Values for the apparent total body clearance of duloxetine from plasma after oral administration (CL/F) in subjects with ESRD were similar to CL/F values in patients with normal renal function or with mild or moderate renal impairment.
Conclusion
Dose adjustments for duloxetine are not necessary for patients with mild or moderate renal impairment (CLcr ≥30 mL/min). For patients with ESRD or severe renal impairment (CLcr <30 mL/min), exposures of duloxetine and its metabolites are expected to increase; therefore, duloxetine is not generally recommended for these patients.









Similar content being viewed by others
References
Wong DT, Bymaster FP, Mayle DA, et al. LY248686, a new inhibitor of serotonin and norepinephrine uptake. Neuropsychopharmacology 1993 Jun; 8: 23–33
Kihara T, Ikeda M. Effects of duloxetine, a new serotonin and norepinephrine uptake inhibitor, on extracellular monoamine levels in rat frontal cortex. J Pharmacol Exp Ther 1995 Jan; 272: 177–83
Kasamo K, Blier P, de Montigny C. Blockade of the serotonin and norepinephrine uptake processes by duloxetine: in vitro and in vivo studies in the rat brain. J Pharmacol Exp Ther 1996 Apr; 277: 278–86
Nemeroff CB, Schatzberg AF, Goldstein DJ, et al. Duloxetine for the treatment of major depressive disorder. Psychopharmacol Bull 2002 Autumn; 36: 106–32
Pinder RM. Designing a new generation of antidepressant drugs. Acta Psychiatr Scand Suppl 1997; 391: 7–13
Pitsikas N. Duloxetine Eli Lilly & Co. Curr Opin Investig Drugs 2000 Sep; 1: 116–21
Raskin J, Smith TR, Wong K, et al. Duloxetine versus routine care in the long-term management of diabetic peripheral neuropathic pain. J Palliat Med 2006 Feb; 9: 29–40
Goldstein DJ, Lu Y, Detke MJ, et al. Duloxetine versus placebo in patients with painful diabetic neuropathy. Pain 2005 Jul; 116: 109–18
Koponen H, Allgulander C, Erickson J, et al. Efficacy of duloxetine for the treatment of generalized anxiety disorder: implications for primary care physicians. Prim Care Companion J Clin Psychiatry 2007; 9: 100–7
Allgulander C, Hartford J, Russell J, et al. Pharmacotherapy of generalized anxiety disorder: results of duloxetine treatment from a pooled analysis of three clinical trials. Curr Med Res Opin 2007 Jun; 23: 1245–52
Yentreve: summary of product characteristics. Basingstoke: Eli Lilly and Company, 2007
van Kerrebroeck P, Abrams P, Lange R, et al. Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. BJOG 2004 Mar; 111: 249–57
Arnold LM, Rosen A, Pritchett YL, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 2005; 119(1–3): 5–15
Cymbalta: summary of product characteristics. Basingstoke: Eli Lilly and Company, 2007
Cymbalta: package insert. Indianapolis (IN): Eli Lilly and Company, 2007
Sharma A, Goldberg MJ, Cerimele BJ. Pharmacokinetics and safety of duloxetine, a dual-serotonin and norepinephrine reuptake inhibitor. J Clin Pharmacol 2000 Feb; 40: 161–7
Lantz RJ, Gillespie TA, Rash TJ, et al. Metabolism, excretion, and pharmacokinetics of duloxetine in healthy human subjects. Drug Metab Dispos 2003 Sep; 31: 1142–50
Skinner MH, Kuan H-Y, Pan A, et al. Duloxetine is both an inhibitor and a substrate of cytochrome P4502D6 in healthy volunteers. Clin Pharmacol Ther 2003 Mar; 73: 170–7
Skinner MH, Kuan H-Y, Skerjanec A, et al. Effect of age on the pharmacokinetics of duloxetine in women. Br J Clin Pharmacol 2004 Jan; 57: 54–61
Suri A, Reddy S, Gonzales C, et al. Duloxetine pharmacokinetics in cirrhotics compared with healthy subjects. Int J Clin Pharmacol Ther 2005 Feb; 43: 78–84
Suri A, Reddy S, Gonzales C, et al. Duloxetine pharmacokinetics in cirrhotics compared with healthy subjects. Int J Clin Pharmacol Ther 2005; 43: 78–8
Lobo ED, Bergstrom RF, Reddy S, et al. In vitro and in vivo evaluations of cytochrome P450 1A2 interactions with duloxetine. Clin Pharmacokinet 2008; 47(3): 191–202
Kuo F, Gillespie TA, Kulanthaivel P, et al. Synthesis and biological activity of some known and putative duloxetine metabolites. Biorg Med Chem Lett 2004; 14(13): 3481–6
Nolin TD, Naud J, Leblond FA, et al. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther 2008 Jun; 83: 898–903
Goldstein DJ, Mallinckrodt C, Lu Y, et al. Duloxetine in the treatment of major depressive disorder: a double-blind clinical trial. J Clin Psychiatry 2002 Mar; 63: 225–31
Raskin J, Goldstein DJ, Mallinckrodt CH, et al. Duloxetine in the long-term treatment of major depressive disorder. J Clin Psychiatry 2003 Oct; 64: 1237–44
Wernicke JF, Pritchett YL, D’souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006 Oct; 67: 1411–20
Bump RC, Voss S, Beardsworth A, et al. Long-term efficacy of duloxetine in women with stress urinary incontinence. BJU Int 2008; 102(2): 214–8
Satonin DK, McCulloch JD, Kuo F, et al. Development and validation of a liquid chromatography-tandem mass spectrometric method for the determination of the major metabolites of duloxetine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2007 Jun; 852: 582–9
Lobo ED, Quinlan T, O’Brien L, et al. Population pharmacokinetics of orally administered duloxetine in patients: implications for dosing recommendation. Clin Pharmacokinet 2009; 48(3): 189–97
US FDA Center for Drug Evaluation and Research [CDER]. Guidance for industry: safety testing of drug metabolites. Rockville (MD): CDER, 2008 Feb [online]. Available from URL: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079266.pdf [Accessed 2010 Feb 19]
Data on file, Eli Lilly and Company, 2009
Australian Government Department of Health and Ageing. PBS for health professionals: duloxetine [online]. Available from URL: http://www.pbs.gov.au/html/healthpro/search/results?term=duloxetine&scope=PBS+STATIC+WEB+NEWS&form-type=simple [Accessed 2010 Mar 2]
Chan C, Yeo KP, Pan AX, et al. Duloxetine pharmacokinetics are similar in Japanese and Caucasian subjects. Br J Clin Pharmacol 2007; 63(3): 310–4
Schrenk D, Brockmeier D, Morike K, et al. A distribution study of CYP1A2 phenotypes among smokers and non-smokers in a cohort of healthy Caucasian volunteers. Eur J Clin Pharmacol 1998; 53(5): 361–7
Wernicke JF, Prakash A, Kajdasz DK, et al. Safety and tolerability of duloxetine treatment of diabetic peripheral neuropathic pain between patients with and without cardiovascular conditions. J Diabetes Complications 2009 Sep–Oct; 23(5): 349–59
Daugirdas JT. Pathophysiology of dialysis hypotension: an update. Am J Kidney Dis 2001; 38(4 Suppl. 4): S11–7
Zhang L, Chappell J, Gonzales CR, et al. QT effects of duloxetine at supratherapeutic doses: a placebo and positive controlled study. J Cardiovasc Pharmacol 2007 Mar; 49: 146–53
Acknowledgements
This study was funded by Eli Lilly and Company (Indianapolis, IN, USA). All authors except for Han-Yi Kuan, PhD, and Michael Skinner, MD, PharmD, are present employees of Eli Lilly and Company. Drs Kuan and Skinner are former employees of Eli Lilly and Company. All authors are stock owners in Eli Lilly and Company. The authors would like to thank all subjects and patients for participating in the clinical trials, Darlene Satonin and Julie McCulloch for analyses of the plasma samples for the two major metabolites, and Jessie Bishop for writing support for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lobo, E.D., Heathman, M., Kuan, HY. et al. Effects of Varying Degrees of Renal Impairment on the Pharmacokinetics of Duloxetine. Clin Pharmacokinet 49, 311–321 (2010). https://doi.org/10.2165/11319330-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11319330-000000000-00000