Abstract
Background: Little is known about how community pharmacists make decisions about which over-the-counter (OTC) medication to supply to a patient and the role of clinical evidence in making those decisions.
Objective: To explore factors that influence product selection by the pharmacist and the role of evidence-based practice in this decision.
Methods: In this qualitative study, community pharmacists registered in Northern Ireland and recruited via advertising and various qualitative sampling techniques, participated in face-to-face, semi-structured interviews (June 2007–September 2007) to discuss issues around OTC medication, including the use of evidence, how they judged a product to be effective, and their views on evidence-based medicine and its application to OTC medication. All interviews were digitally recorded, fully transcribed and analysed using the principles of constant comparison.
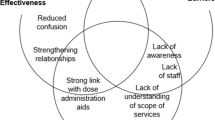
Results: Twenty-six pharmacists participated in interviews. Safety was the overarching consideration for pharmacists when making decisions. The subordinate themes were product, patient and professional factors. In terms of the product subordinate theme, use or consideration of evidence was secondary in the selection of OTC medicines. Pharmacists considered the potential for harm in the first instance and if the product was deemed safe, although lacking any evidence for effectiveness, the product was supplied. In relation to patient factors, it emerged that pharmacists were influenced by patient demand for a particular OTC product and wanted to meet patient expectations, provided that the requested product was judged to be safe. Similarly, professional factors such as ethical considerations (primarily in relation to safety) and respecting patient choice also influenced decision making. However, pharmacists recognized the conflict between professional requirements to practise according to evidence-based principles and patient demands.
Conclusion: This study suggests that pharmacists considered safety above all other factors when recommending OTC products to patients, and evidence of effectiveness was seldom considered when selling OTC medicines. If evidence-based practice is to influence this type of decision, pharmacists need to use the evidence that is available and be prepared to discuss evidence with patients.


Similar content being viewed by others
References
Blenkinsopp A, Bond C. Over-the-counter medication. London: British Medical Association, 2005
The Association of the British Pharmaceutical Industry. Understanding the pharmaceutical price regulation scheme. London: The Association of the British Pharmaceutical Industry, 2005
The Medicines and Healthcare products Regulatory Agency (MHRA). Flomax relief MR (PL 00015/0280) UKPAR [online]. Available from URL: http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con068200.pdf [Accessed 2010 Feb 3]
The Medicines and Healthcare products Regulatory Agency (MHRA). Recent reclassifications [online]. Available from URL: http://www.mhra.gov.uk [Accessed 2009 Sep 28]
The Royal Pharmaceutical Society of Great Britain (RPSGB). Potential candidates for reclassification from POM to P [online]. Available from URL: http://www.rpsgb.org.uk/pdfs/pomtopreclasslist.pdf [Accessed 2009 Mar 27]
Department of Health. Promoting clinical effectiveness: a framework for action in and through the NHS. London: Department of Health, 1996
Sackett DL, Rosenberg WMC, Gray JM, et al. Evidence based medicine: what it is and what it isn’t. BMJ 1996; 312: 71–2
Royal Pharmaceutical Society of Great Britain. Medicines, ethics and practice: a guide for pharmacists and pharmacy technicians. Issue 33. London: Pharmaceutical Press, 2009 Jul
Wazaify M, Hughes CM, McElnay JC. The implementation of a harm minimization model for the identification and treatment of over-the-counter drug misuse and abuse in community pharmacies in Northern Ireland. Patient Educ Couns 2006; 64: 136–41
Smith MB, Feldman W. Over-the-counter cold medications: a critical review of clinical trials between 1950 and 1992. JAMA 1993; 269: 2258–63
Smith SM, Schroeder K, Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev 2008; (1): CD001831
Pray WS. Ethical, scientific, and educational concerns with unproven medications. Am J Pharm Edu 2006; 70(6): 141
Colquhoun D. Science degrees without the science. Nature 2007; 446: 373–4
Naidu S, Wilkinson JM, Simpson MD. Attitudes of Australian pharmacists toward complementary and alternative medicines. Ann Pharmacother 2005; 39: 1456–61
Ernst E. How much of CAM is based on research evidence? Evid Based Complement Alternat Med. Epub 2009 May 21
Ernst E. Should we use “powerful placebos”? Pharm J 2004; 273: 795
Miller F, Rosenstein D. The nature and power of the placebo effect. J Clin Epi 2006; 59: 331–5
Rutter P. Community pharmacy: symptoms, diagnosis and treatment. 2nd ed. Edinburgh: Churchill Livingstone Elsevier, 2009
Blenkinsopp A, Paxton P, Blenkinsopp J. Symptoms in the pharmacy: a guide to the management of common illness. 6th ed. Chichester: Wiley-Blackwell, 2009
Edwards C, Stillman P. Minor illness or major disease? The clinical pharmacist in the community. 4th ed. London: Pharmaceutical Press, 2006
Nathan A. Non-prescription medicines. 3rd ed. London: Pharmaceutical Press, 2006
Britten N. Qualitative research: qualitative interviews in medical research. BMJ 1995; 311: 251–3
Mays N, Pope C. Qualitative research: rigour and qualitative research. BMJ 1995; 311: 109–12
Marshall MN. Sampling for qualitative research. Fam Pract 1996; 13: 522–5
Sandelowski M. Focus on research methods combining qualitative and quantitative sampling, data collection, and analysis techniques in mixed-method studies. Res Nurs Health 2000; 23: 246–55
Tracy CS, Dantas GC, Upshur REG. Evidence-based medicine in primary care: qualitative study of family physicians. BMC Fam Pract 2003; 4: 6–14
Donovan J, Mills N, Smith M, et al. Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (Prostate Testing for Cancer and Treatment) study. BMJ 2002; 325: 766–70
Spencer L, Ritchie J, O’Connor W. Analysis: practices, principles and processes. In: Ritchie J, Lewis J, editors. Qualitative research practice. London: Sage Publications, 2004: 199–218
Mays N, Pope C. Qualitative research in health care: assessing quality in qualitative research. BMJ 2000; 320: 114–6
Watson MC, Bond CM. The evidence-based supply of non-prescription medicines: barriers and beliefs. Int J Pharm Pract 2004; 12: 65–72
Department of Health. Building on the best: choice, responsiveness and equity in the NHS. London: Department of Health, 2003 Dec
Parliamentary Office of Science and Technology. Changing role of pharmacies. Postnote 2005 Jul, No. 246 [online]. Available from URL: http://www.parliament.uk/documents/upload/postpn246.pdf [Accessed 2010 Jan 29]
Department of Health. The NHS plan: a plan for investment, a plan for reform. Presented to Parliament by the Secretary of State for Health by Command of Her Majesty, 2000 Jul [online]. Available from URL: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyandGuidance/DH_4002960 [Accessed 2009 Dec 29]
Shang A, Huwiler-Muntener K, Nartey L, et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy. Lancet 2005; 366: 726–32
Stevenson FA, Greenfield SM, Jones M, et al. GPs’ perceptions of patient influence on prescribing. Fam Pract 1999; 16: 255–61
Bradley CP. Uncomfortable prescribing decisions: a critical incident study. BMJ 1992; 304: 294–6
Ferner RE, Beard K. Over the counter medicines: proceed with caution. BMJ 2008; 336: 694–6
Tracy CS, Dantas GC, Moineddin R, et al. The nexus of evidence, context and patient preferences in primary care: postal survey of Canadian family physicians. BMC Fam Pract 2003; 4: 13–20
Marinker M, editor. From compliance to concordance: achieving shared goals in medicine taking. London: Royal Pharmaceutical Society of Great Britain and Merck Sharp & Dohme, 1997
Britten N, Weiss M. What is concordance? In: Bond CM, editor. Concordance. London: The Pharmaceutical Press, 2004: 9–28
Ring A, Dowrick C, Humphris G, et al. Do patients with unexplained physical symptoms pressurize general practitioners for somatic treatment? A qualitative study. BMJ 2004; 328: 1057–60
Emmerton L, Shaw J. The influence of pharmacy staffing in non-prescription medicine sales. Int J Pharm Pract 2002; 10: 101–6
Watson MC, Bond CM, Grimshaw JM, et al. Factors predicting the guideline compliant supply (or non-supply) of non-prescription medicines in the community pharmacy setting. Qual Saf Health Care 2006; 15: 53–7
Seston E, Nicolson M, Hassell K, et al. ‘Not just someone stood behind the counter’. The views and experiences of medicines counter assistants. J Soc Admin Pharm 2001; 18: 122–8
Bissell P, Ward P, Noyce P. Appropriateness measurement: application to advice-giving in community pharmacies. Soc Sci Med 2000; 51: 343–59
Ernst E. Is it ethical for pharmacists to sell unproven or disproven medicines? Pharm J 2008; 281: 75–6
Maguire T. We are what we sell: questionable quality and efficacy of slimming aids. Pharm J 2008; 281: 96
O’Donnell CA. Attitudes and knowledge of primary care professionals towards evidence-based practice: a postal survey. J Eval Clin Pract 2004; 10: 197–2005
Sharfstein JM, North M, Serwint JR. Over the counter but no longer under the radar: pediatric cough and cold medications. N Engl J Med 2007; 357: 2321–4
The Medicines and Healthcare products Regulatory Agency (MHRA). Children’s over-the-counter cough and cold medicines: new advice [online]. Available from URL: http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON038908 [Accessed 2009 Mar 27]
Malterud K. Qualitative research: standards, challenges and guidelines. Lancet 2001; 358: 483–8
Acknowledgements
The authors wish to acknowledge all the pharmacists who participated in this study and Ms Aileen Hamilton who transcribed all the recordings. This study was supported through funding from the Northern Ireland Centre for Pharmacy Learning and Development, and Queen’s University Belfast Staff Training and Development Unit. The funding organizations had no part in any aspects of the study from conception through to final analysis and production of this study. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanna, LA., Hughes, C.M. ‘First, Do No Harm’: Factors that Influence Pharmacists Making Decisions about Over-the-Counter Medication. Drug-Safety 33, 245–255 (2010). https://doi.org/10.2165/11319050-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11319050-000000000-00000