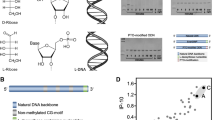
Abstract
Toll-like receptors (TLRs) are part of the innate immune system, and they belong to the pattern recognition receptors (PRR) family. The PRR family is designed to recognize and bind conserved pathogen-associated molecular patterns, which are not generated by the host and are restricted and essential to microorganisms. TLR9, which recognizes unmethylated CpG (cytosine guanosine dinucleotide), is a very promising target for therapeutic activation. Stimulation of TLR9 activates human plasmacytoid dendritic cells and B cells, and results in potent T helper-1 (Th1)-type immune responses and antitumor responses in mouse tumor models and in patients.
Several pharmaceutical companies, such as Pfizer, Idera, and Dynavax, are developing CpG oligodeoxynucleotides (ODNs) for the treatment of cancer, along with other conditions, such as infections and allergy. CpG ODNs have shown promising results as vaccine adjuvants and in combination with cancer immunotherapy. Several TLR9 agonists are being developed and have entered clinical trials to evaluate their safety and efficacy for the treatment of several hematopoietic and solid tumors. In this review, we discuss the use of CpG ODNs in several phase I and II clinical trials for the treatment of NHL, renal cell carcinoma, melanoma, and non-small cell lung cancer, either alone or in combination with other agents.

Similar content being viewed by others
References
Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature 1996 10/31; 383(6603): 787–93
Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003; 21: 335–76
Medzhitov R, Preston-Hurlburt P, Janeway Jr CA, et al. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997; 388(6640): 394–7
Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001 08; 2(8): 675–80
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004 07; 4(7): 499–511
Akira S. TLR signaling: from innate immunity to immunological memory. Curr Top Microbiol Immunol 2006; 311: 1–16
Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004 10; 5(10): 987–95
Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther 1994; 64(3): 529–64
Krieg AM. CpG motifs in bacterial DNA and their immune effects. Ann Rev Immunol 2002; 20: 709–60
Tokunaga T, Yamamoto H, Shimada S, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG I: isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst 1984 Apr; 72(4): 955–62
Yamamoto S, Kuramoto E, Shimada S, et al. In vitro augmentation of natural killer cell activity and production of interferon-alpha/beta and -gamma with deoxyribonucleic acid fraction from Mycobacterium bovis BCG. Jpn J Cancer Res 1988 Jul; 79(7): 866–73
Meyer T, Stockfleth E. Clinical investigations of Toll-like receptor agonists. Expert Opin Investig Drugs 2008 Jul; 17(7): 1051–65
Bauer S, Kirschning CJ, Hacker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A 2001 Jul 31; 98(16): 9237–42
Wenzel J, Tormo D, Tuting T. Toll-like receptor-agonists in the treatment of skin cancer: history, current developments and future prospects. Handbook Exper Pharmacol 2008; (183): 201-20
Leifer CA, Kennedy MN, Mazzoni A, et al. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol 2004 Jul 15; 173(2): 1179–83
Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nature Immunol 2004 Feb; 5(2): 190–8
Ahmad-Nejad P, Hacker H, Rutz M, et al. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol 2002 Jul; 32(7): 1958–68
Yi AK, Chang M, Peckham DW, et al. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol 1998 Jun 15; 160(12): 5898–906
Rankin R, Pontarollo R, Ioannou X, et al. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Devel 2001 Oct; 11(5): 333–40
Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995 Apr 6; 374(6522): 546–9
Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol 2000 Jan 15; 164(2): 944–53
Uhlmann E, Vollmer J. Recent advances in the development of immunostimulatory oligonucleotides. Curr Opin Drug Discov Develop 2003 Mar; 6(2): 204–17
Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov 2006 06; 5(6): 471–84
Krug A, Rothenfusser S, Selinger S, et al. CpG-A oligonucleotides induce a monocyte-derived dendritic cell-like phenotype that preferentially activates CD8 T cells. J Immunol 2003 Apr 1; 170(7): 3468–77
Kerkmann M, Costa LT, Richter C, et al. Spontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-alpha induction by CpG-A in plasmacytoid dendritic cells. J Biol Chem 2005 Mar 4; 280(9): 8086–93
Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol 2001 Oct; 31(10): 3026–37
Marshall JD, Fearon K, Abbate C, et al. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J Leukoc Biol 2003 Jun; 73(6): 781–92
Hartmann G, Battiany J, Poeck H, et al. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur J Immunol 2003 Jun; 33(6): 1633–41
Leifer CA, Brooks JC, Hoelzer K, et al. Cytoplasmic targeting motifs control localization of toll-like receptor 9. J Biol Chem 2006 Nov 17; 281(46): 35585–92
Kim YM, Brinkmann MM, Paquet ME, et al. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 2008 Mar 13; 452(7184): 234–8
Chockalingam A, Brooks JC, Cameron JL, et al. TLR9 traffics through the Golgi complex to localize to endolysosomes and respond to CpG DNA. Immunol Cell Biol 2008 Mar–Apr; 87(3): 209–17
Hacker H, Mischak H, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J 1998; 17(21): 6230–40
Ewald SE, Lee BL, Lau L, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 2008 Dec 4; 456(7222): 658–62
Lamphier MS, Sirois CM, Verma A, et al. TLR9 and the recognition of self and non-self nucleic acids. Ann N Y Acad Sci 2006 Oct; 1082: 31–43
Yasuda K, Rutz M, Schlatter B, et al. CpG motif-independent activation of TLR9 upon endosomal translocation of ‘natural’ phosphodiester DNA. Eur J Immunol 2006 Feb; 36(2): 431–6
Krieg AM. Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep 2004 Mar; 6(2): 88–95
Krug A, Rothenfusser S, Hornung V, et al. Identification of CpG oligonu-cleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol 2001 Jul; 31(7): 2154–63
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev 2004 Jul; 4(7): 499–511
West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Ann Rev Cell Dev Biol 2006; 22: 409–37
Krieg AM. CpG motifs in bacterial DNA and their immune effects. Ann Rev Immunol 2002; 20: 709–60
Hacker H, Vabulas RM, Takeuchi O, et al. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF) 6. J Exp Med 2000; 192(4): 595–600
Hacker H. Signal transduction pathways activated by CpG-DNA. Curr Top Microbiol Immunol 2000; 247: 77–92
Park JE, Kim YI, Yi AK. Protein kinase D1: a new component in TLR9 signaling. J Immunol 2008 Aug 1; 181(3): 2044–55
Younis HS, Vickers T, Levin AA, et al. CpG and Non-CpG oligodeoxynucleotides induce differential proinflammatory gene expression profiles in liver and peripheral blood leukocytes in mice. J Immunotoxicol 2006 Jul 1; 3(2): 57–68
Kato A, Homma T, Batchelor J, et al. Interferon-alpha/beta receptor-mediated selective induction of a gene cluster by CpG oligodeoxynucleotide 2006. BMC Immunol 2003 Jul 30; 4: 8
Welner RS, Pelayo R, Nagai Y, et al. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood 2008 Nov 1; 112(9): 3753–61
van Ojik HH, Bevaart L, Dahle CE, et al. CpG-A and B oligodeoxynucleotides enhance the efficacy of antibody therapy by activating different effector cell populations. Cancer Res 2003 Sep 1; 63(17): 5595–600
Paget C, Mallevaey T, Speak AO, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 2007 Oct; 27(4): 597–609
Uematsu S, Akira S. Toll-like receptors and type I interferons. J Biol Chem 2007 May 25; 282(21): 15319–23
Horkheimer I, Quigley M, Zhu J, et al. Induction of type I IFN is required for overcoming tumor-specific T cell tolerance following stem cell transplantation. Blood 2009 May 21; 113(21): 5330–9
Ren T, Wen ZK, Liu ZM, et al. Targeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances anti-tumor responses of peripheral blood mononuclear cells from human lung cancer patients. Cancer Invest 2008 Jun; 26(5): 448–55
Haining WN, Davies J, Kanzler H, et al. CpG oligodeoxynucleotides alter lymphocyte and dendritic cell trafficking in humans. Clin Cancer Res 2008 Sep 1; 14(17): 5626–34
Ding C, Wang L, Marroquin J, et al. Targeting of antigens to B cells augments antigen-specific T-cell responses and breaks immune tolerance to tumor-associated antigen MUC1. Blood 2008 Oct 1; 112(7): 2817–25
Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 2002 Dec 13; 298(5601): 2199–202
Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood 2003 Jun 1; 101(11): 4500–4
Jung J, Yi AK, Zhang X, et al. Distinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNA. J Immunol 2002 Sep 1; 169(5): 2368–73
Richard K, Pierce SK, Song W. The agonists of TLR4 and 9 are sufficient to activate memory B cells to differentiate into plasma cells in vitro but not in vivo. J Immunol 2008 Aug 1; 181(3): 1746–52
Leadbetter EA, Rifkin IR, Hohlbaum AM, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 2002 Apr 11; 416(6881): 603–7
Viglianti GA, Lau CM, Hanley TM, et al. Activation of autoreactive B cells by CpG dsDNA. Immunity 2003 Dec; 19(6): 837–47
O'Neill SK, Veselits ML, Zhang M, et al. Endocytic sequestration of the B cell antigen receptor and toll-like receptor 9 in anergic cells. Proc Natl Acad Sci U S A 2009 Apr 14; 106(15): 6262–7
Jegerlehner A, Maurer P, Bessa J, et al. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol 2007 Feb 15; 178(4): 2415–20
Jiang W, Lederman MM, Harding CV, et al. TLR9 stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol 2007 Aug; 37(8): 2205–13
Eckl-Dorna J, Batista FD. BCR-mediated uptake of antigen linked to TLR9-ligand stimulates B-cell proliferation and antigen-specific plasma cell formation. Blood 2009 Apr 23; 113(17): 3969–77
Decker T, Schneller F, Sparwasser T, et al. Immunostimulatory CpG-oligonucleotides cause proliferation, cytokine production, and an immunogenic phenotype in chronic lymphocytic leukemia B cells. Blood 2000 Feb 1; 95(3): 999–1006
Jahrsdorfer B, Hartmann G, Racila E, et al. CpG DNA increases primary malignant B cell expression of costimulatory molecules and target antigens. J Leukocyte Biol 2001 Jan; 69(1): 81–8
Asselin-Paturel C, Boonstra A, Dalod M, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol 2001 12; 2(12): 1144–50
Faith A, Peek E, McDonald J, et al. Plasmacytoid dendritic cells from human lung cancer draining lymph nodes induce Tc1 responses. Am J Respir Cell Mol Biol 2007 Mar; 36(3): 360–7
Merck Serono. Merck KGaA and Idera Pharmaceuticals to collaborate on development of TLR9 agonists for treatment of cancer [online]. Available from URL: http://www.merckserono.com/corp.merckserono/en/images/20071219_en_tcm112_16861.pdf [Accessed 2009 Oct 1]
Damiano V, Caputo R, Bianco R, et al. Novel toll-like receptor 9 agonist induces epidermal growth factor receptor (EGFR) inhibition and synergistic antitumor activity with EGFR inhibitors. Clin Cancer Res 2006 Jan 15; 12(2): 577–83
Damiano V, Caputo R, Garofalo S, et al. TLR9 agonist acts by different mechanisms synergizing with bevacizumab in sensitive and cetuximab-resistant colon cancer xenografts. Proc Natl Acad Sci U S A 2007 Jul 24; 104(30): 12468–73
Zeromski J, Mozer-Lisewska I, Kaczmarek M. Significance of Toll-like receptors expression in tumor growth and spreading: a short review. Cancer Microenviron 2008 Dec; 1(1): 37–42
Kundu SD, Lee C, Billips BK, et al. The toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cells. Prostate 2008 Feb 1; 68(2): 223–9
Merrell MA, Ilvesaro JM, Lehtonen N, et al. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol Cancer Res 2006 Jul; 4(7): 437–47
Ilvesaro JM, Merrell MA, Swain TM, et al. Toll like receptor-9 agonists stimulate prostate cancer invasion in vitro. Prostate 2007 May 15; 67(7): 774–81
Coussens LM, Werb Z. Inflammation and cancer. Nature 2002 Dec 19-26; 420(6917): 860–7
Ren T, Xu L, Jiao S, et al. TLR9 Signaling promotes tumor progression of human lung cancer cell in vivo. Pathol Oncol Res. Epub 2009 Mar 25
Monteith DK, Levin AA. Synthetic oligonucleotides: the development of antisense therapeutics. Toxicol Pathol 1999 Jan–Feb; 27(1): 8–13
Levin AA. A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim Biophys Acta 1999 Dec 10; 1489(1): 69–84
Geary RS, Leeds JM, Henry SP, et al. Antisense oligonucleotide inhibitors for the treatment of cancer 1: pharmacokinetic properties of phosphorothioate oligodeoxynucleotides. Anticancer Drug Des 1997 Jul; 12(5): 383–93
Cossum PA, Sasmor H, Dellinger D, et al. Disposition of the 14C-labeled phosphorothioate oligonucleotide ISIS 2105 after intravenous administration to rats. J Pharmacol Exp Ther 1993 Dec; 267(3): 1181–90
Link BK, Ballas ZK, Weisdorf D, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother 2006 Sep-Oct; 29(5): 558–68
Henry SP, Beattie G, Yeh G, et al. Complement activation is responsible for acute toxicities in rhesus monkeys treated with a phosphorothioate oligodeoxynucleotide. Int Immunopharmacol 2002 Nov; 2(12): 1657–66
Galbraith WM, Hobson WC, Giclas PC, et al. Complement activation and hemodynamic changes following intravenous administration of phosphorothioate oligonucleotides in the monkey. Antisense Res Dev 1994 Fall; 4(3): 201–6
Henry SP, Novotny W, Leeds J, et al. Inhibition of coagulation by a phosphorothioate oligonucleotide. Antisense Nucleic Acid Drug Dev 1997 Oct; 7(5): 503–10
Ichikawa HT, Williams LP, Segal BM. Activation of APCs through CD40 or Toll-like receptor 9 overcomes tolerance and precipitates autoimmune disease. J Immunol 2002 Sep 1; 169(5): 2781–7
Ronaghy A, Prakken BJ, Takabayashi K, et al. Immunostimulatory DNA sequences influence the course of adjuvant arthritis. J Immunol 2002 Jan 1; 168(1): 51–6
Moseman EA, Liang X, Dawson AJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol 2004 Oct 1; 173(7): 4433–42
Becker Y. CpG ODNs treatments of HIV-1 infected patients may cause the decline of transmission in high risk populations: a review, hypothesis and implications. Virus Genes 2005 Mar; 30(2): 251–66
Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nature Rev 2006 Jun; 5(6): 471–84
Boule MW, Broughton C, Mackay F, et al. Toll-like receptor 9-dependent and-independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med 2004 Jun 21; 199(12): 1631–40
Herlands RA, Christensen SR, Sweet RA, et al. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity 2008 Aug; 29(2): 249–60
Ishii KJ, Gursel I, Gursel M, et al. Immunotherapeutic utility of stimulatory and suppressive oligodeoxynucleotides. Curr Opin Mol Ther 2004 Apr; 6(2): 166–74
Krieg AM, Efler SM, Wittpoth M, et al. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligo-deoxynucleotide TLR9 agonist. J Immunother 2004 11; 27(6): 460–71
Bourquin C, Anz D, Zwiorek K, et al. Targeting CpG oligonucleotides to the lymph node by nanoparticles elicits efficient antitumoral immunity. J Immunol 2008 Sep 1; 181(5): 2990–8
Najar HM, Dutz JP. Topical TLR9 agonists induce more efficient cross-presentation of injected protein antigen than parenteral TLR9 agonists do. Eur J Immunol 2007 Aug; 37(8): 2242–56
Chuang CM, Monie A, Wu A, et al. Treatment with LL-37 peptide enhances the antitumor effects induced by CpG oligodeoxynucleotides against ovarian cancer. Hum Gene Ther 2009 Apr; 20(4): 303–13
Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000 Dec 7; 408(6813): 740–5
Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev 2008 Apr 29; 60(7): 795–804
Ballas ZK, Krieg AM, Warren T, et al. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol 2001 Nov 1; 167(9): 4878–86
Carpentier AF, Chen L, Maltonti F, et al. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res 1999 Nov 1; 59(21): 5429–32
Lonsdorf AS, Kuekrek H, Stern BV, et al. CpG-oligodeoxynucleotide injection induces protective antitumor T cell immunity. J Immunol 2003 Oct 15; 171(8): 3941–6
Baines J, Celis E. Immune-mediated tumor regression induced by CpG-containing oligodeoxynucleotides. Clin Cancer Res 2003 Jul; 9(7): 2693–700
Heckelsmiller K, Rall K, Beck S, et al. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol 2002 Oct 1; 169(7): 3892–9
Kawarada Y, Ganss R, Garbi N, et al. NK- and CD8(+) T cell-mediated eradication of established tumors by peritumoral injection of CpG-containing oligodeoxynucleotides. J Immunol 2001 Nov 1; 167(9): 5247–53
Mastini C, Becker PD, Iezzi M, et al. Intramammary application of non-methylated-CpG oligodeoxynucleotides (CpG) inhibits both local and systemic mammary carcinogenesis in female BALB/c Her-2/neu transgenic mice. Curr Cancer Drug Targets 2008 May; 8(3): 230–42
Wang XS, Sheng Z, Ruan YB, et al. CpG oligodeoxynucleotides inhibit tumor growth and reverse the immunosuppression caused by the therapy with 5-fluorouracil in murine hepatoma. World J Gastroenterol 2005 Feb 28; 11(8): 1220–4
Balsari A, Tortoreto M, Besusso D, et al. Combination of a CpG-oligodeoxynucleotide and a topoisomerase I inhibitor in the therapy of human tumour xenografts. Eur J Cancer 2004 May; 40(8): 1275–81
Weigel BJ, Rodeberg DA, Krieg AM, et al. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin Cancer Res 2003 Aug 1;9(8): 3105–14
Weeratna RD, Bourne LL, Sullivan SM, et al. Combination of a new TLR9 agonist immunomodulator (CpG 7909) and paclitaxel for treatment of metastatic Lewis Lung Carcinoma (LLC). ASCO Meeting Abstracts 2004 Jul 15;22(14Suppl.): 7346
Bourquin C, Schreiber S, Beck S, et al. Immunotherapy with dendritic cells and CpG oligonucleotides can be combined with chemotherapy without loss of efficacy in a mouse model of colon cancer. Int J Cancer 2006 Jun 1; 118(11): 2790–5
Kim SK, Ragupathi G, Musselli C, et al. Comparison of the effect of different immunological adjuvants on the antibody and T-cell response to immunization with MUC1-KLH and GD3-KLH conjugate cancer vaccines. Vaccine 1999 Nov 12; 18(7-8): 597–603
Chu RS, Targoni OS, Krieg AM, et al. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med 1997 Nov 17; 186(10): 1623–31
He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol 2004 Oct 1; 173(7): 4479–91
Mukherjee P, Pathangey LB, Bradley JB, et al. MUC1-specific immune therapy generates a strong anti-tumor response in a MUC1-tolerant colon cancer model. Vaccine 2007 Feb 19; 25(9): 1607–18
Zheng R, Cohen PA, Paustian CA, et al. Paired Toll-like receptor agonists enhance vaccine therapy through induction of interleukin-12. Cancer Res 2008 Jun 1; 68(11): 4045–9
Weeratna R, Comanita L, Davis HL. CPG ODN allows lower dose of antigen against hepatitis B surface antigen in BALB/c mice. Immunol Cell Biol 2003 Feb; 81(1): 59–62
Davis HL, Weeratna R, Waldschmidt TJ, et al. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol 1998 Jan 15; 160(2): 870–6
Halperin SA, Van Nest G, Smith B, et al. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine 2003 Jun 2; 21(19-20): 2461–7
Verthelyi D, Kenney RT, Seder RA, et al. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J Immunol 2002 Feb 15; 168(4): 1659–63
Davis HL, Suparto II, Weeratna RR, et al. CpG DNA overcomes hypo-responsiveness to hepatitis B vaccine in orangutans. Vaccine 2000 Mar 17; 18(18): 1920–4
Leavitt RD, Ratanatharathorn V, Ozer H, et al. Alfa-2b interferon in the treatment of Hodgkin's disease and non-Hodgkin's lymphoma. Semin Oncol 1987 Jun; 14(2 Suppl. 2): 18–23
McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 1998 Aug; 16(8): 2825–33
Duggan DB, Santarelli MT, Zamkoff K, et al. A phase II study of recombinant interleukin-2 with or without recombinant interferon-beta in non-Hodgkin's lymphoma: a study of the Cancer and Leukemia Group B. J Immunother (1991) 1992 Aug; 12(2): 115–22
Wooldridge BKLJ, Weisdorf DJ, Ballas ZK, et al. Phase I study of oligodeoxy-nucleotide CpG 7909 in patients with previously treated non-Hodgkin's lymphoma [abstract]. Proc Am Soc Clin Oncol 2003; 22: 843
Wooldridge JE, Ballas Z, Krieg AM, et al. Immunostimulatory oligodeoxynucleotides containing CpG motifs enhance the efficacy of monoclonal antibody therapy of lymphoma. Blood 1997 Apr 15; 89(8): 2994–8
Jahrsdorfer B, Muhlenhoff L, Blackwell SE, et al. B-cell lymphomas differ in their responsiveness to CpG oligodeoxynucleotides. Clin Cancer Res 2005 Feb 15; 11(4): 1490–9
Leonard JP, Link BK, Emmanouilides C, et al. Phase I trial of toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin's lymphoma. Clin Cancer Res 2007 Oct 15; 13(20): 6168–74
Weiner GJ, Link BK, Leonard J, et al. Combination of CpG7909 and rituximab in patients with relapsed or refractory B-cell non-Hodgkin's lymphoma (NHL): a phase I, open label dose-escalation study of safety and tolerability. ASCO Meeting Abstracts 2004 Jul 15; 22(14 Suppl.): 6594
Friedberg JW, Kim H, McCauley M, et al. Combination immunotherapy with a CpG oligonucleotide 1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-alpha/beta-inducible gene expression, without significant toxicity. Blood 2005 Jan 15; 105(2): 489–95
National Institutes of Health. Phase I/IICPG 7909 + local XRT in recurrent low-grade lymphomas [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00185965?term=nct00185965&rank=1 [Accessed 2009 Oct 1]
Thompson JA, Kuzel T, Bukowski R, et al. Phase Ib trial of a targeted TLR9 CpG immunomodulator (CPG 7909) in advanced renal cell carcinoma (RCC). ASCO Meeting Abstracts 2004 Jul 15; 22(14 Suppl.): 4644
Van Den Eertwegh AJ, Lensen RJ, Scheper RJ, et al. Autologous tumor cell vaccination with PF-3512676 (CPG 7909) and GM-CSF followed by subcutaneous PF-3512676 and IFN-alpha for patients with metastatic renal cell carcinoma. ASCO Meeting Abstracts 2006 Jun 20; 24(18 Suppl.): 2530
Hwang JJ, Park S, Amin A, et al. A phase I study of HYB2055 in patients (pts) with advanced solid malignancies. ASCO Meeting Abstracts 2004 Jul 15; 22(14 Suppl.): 3111
National Institutes of Health. Study of IMO-2055 in metastatic or locally recurrent clear cell renal carcinoma [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00729053?term=NCT00729053&rank=1 [Accessed 2009 Oct 1]
Kirkwood JM, Tarhini AA, Panelli MC, et al. Next generation of immunotherapy for melanoma. J Clin Oncol 2008 Jul 10; 26(20): 3445–55
Pashenkov M, Goess G, Wagner C, et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol 2006 Dec 20; 24(36): 5716–24
Wagner SN, Pashenkov M, Goess G, et al. TLR9-targeted CpG immunostimulatory treatment of metastatic melanoma: a phase II trial with CpG 7909 (ProMune). ASCO Meeting Abstracts 2004 Jul 15; 22(14 Suppl.): 7513
Wagner S, Weber J, Redman B, et al. CPG 7909, a TLR9 agonist immunomodulator in metastatic melanoma: a randomized phase II trial comparing two doses and in combination with DTIC. ASCO Meeting Abstracts 2005 Jun 1; 23(16 Suppl.): 7526
Fourcade J, Kudela P, Andrade Filho PA, et al. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J Immunother 2008 Oct; 31 (8): 781–91
Speiser DE, Lienard D, Rufer N, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 2005 Mar; 115(3): 739–46
National Institutes of Health. Immunization with the MAGE-3.A1 peptide mixed with the adjuvant CpG 7909 in patients with metastatic melanoma [online]. Available from URL: http://www.clinicaltrials.gov/ct2/show/NCT00145145?term=NCT00145145&rank=1 [Accessed 2009 Oct 1]
National Institutes of Health. Safety of adding IMO-2055 to erlotinib + bevacizumab in 2nd line treatment for patients with NSCLC [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00633529?term=NCT00633529&rank=1 [Accessed 2009 Oct 1]
Manegold C, Gravenor D, Woytowitz D, et al. Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol 2008 Aug 20; 26(24): 3979–86
National Institutes of Health. Randomized trial of gemcitabine/cisplatin + PF-3512676 vs gemcitabine/cisplatin alone in patients with advanced NSCLC [online]. Available from URL: http://clinicaltrials.gov/ct2/results?term=NCT00254904 [Accessed 2009 Oct 1]
National Institutes of Health. Trial of paclitaxel/carboplatin + PF-3512676 vs paclitaxel/carboplatin alone in patients with advanced non-small cell lung cancer [online]. Available from URL: http://clinicaltrials.gov/ct2/results?term=NCT00254891 [Accessed 2009 Oct 1]
Hofmann MA, Kors C, Audring H, et al. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother 2008 Jun; 31(5): 520–7
Pratesi G, Petrangolini G, Tortoreto M, et al. Therapeutic synergism of gemcitabine and CpG-oligodeoxynucleotides in an orthotopic human pancreatic carcinoma xenograft. Cancer Research 2005 Jul 15; 65(14): 6388–93
Wang H, Rayburn ER, Wang W, et al. Chemotherapy and chemosensitization of non-small cell lung cancer with a novel immunomodulatory oligonucleotide targeting Toll-like receptor 9. Mol Cancer Ther 2006 Jun; 5(6): 1585–92
National Institutes of Health. Randomized Ph 2 trial of paclitaxel/carboplatin/bevacizumab+PF-3512676 and P/C/B alone in advanced nonsquamous NSCLC [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00313768?term=NCT00313768&rank=1 [Accessed 2009 Oct 1]
National Institutes of Health. Trial of erlotinib with or without PF-3512676 in advanced non small cell lung cancer [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00321815?term=NCT00321815&rank=1 [Accessed 2009 Oct 1]
National Institutes of Health. Trial of pemetrexed with or without PF-3512676 in advanced non-small cell lung cancer [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00321308?term=NCT00321308&rank=1 [Accessed 2009 Oct 1]
Idera Pharmaceuticals. IMO 2055 [online]. Available from URL: http://iderapharma.com/pipeline/imo-2055.php [Accessed 2009 Oct 1]
Wingender G, Garbi N, Schumak B, et al. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur J Immunol 2006 Jan; 36(1): 12–20
Vicari AP, Luu R, Zhang N, et al. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother 2009 Apr; 58(4): 615–28
Acknowledgments
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murad, Y.M., Clay, T.M. CpG Oligodeoxynucleotides as TLR9 Agonists. BioDrugs 23, 361–375 (2009). https://doi.org/10.2165/11316930-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11316930-000000000-00000