Abstract
Background/aims: Non-alcoholic fatty liver disease (NAFLD), the major hepatic manifestation of type 2 diabetes mellitus, is the most common liver disease in the US. Thiazolidinediones, a commonly used drug class for the treatment of type 2 diabetes, have emerged as a potentially useful treatment for NAFLD. There are, however, lingering concerns about their potential toxicity as well as emerging concerns about how to monitor for and assess hepatotoxicity. We conducted a randomized, long-term, double-blind, hepatic safety study at 171 centres in the US in which 2097 patients with type 2 diabetes received either pioglitazone or glibenclamide (glyburide).
Methods: Patients were randomized to receive either pioglitazone (15–45 mg once daily) or glibenclamide (5–15 mg once daily) for 3 years. The primary objective was to evaluate drug-induced liver injury manifested by liver enzyme elevations, measured every 8 weeks for the first year and every 12 weeks thereafter. The primary endpoint was a confirmed ALT greater than three times the upper limit of normal (>3 × ULN) with a secondary endpoint of 8×ULN.
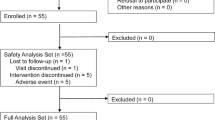
Main results: The intent-to-treat population included 1051 pioglitazonetreated and 1046 glibenclamide-treated patients; of these, 411 pioglitazone patients and 413 glibenclamide patients completed the study. The incidence of hepatocellular injury was 0 with pioglitazone and 4 (0.38%) with glibenclamide (p = 0.0617). Analyses of the secondary endpoints revealed no ALT >8 ×ULN for pioglitazone versus 1 with glibenclamide (p = 0.4988); no ALT >3 × ULN + total bilirubin 2 × ULN with pioglitazone versus 1 with glibenclamide (p = 0.4988); and fewer ALT >3 × ULN single elevations with pioglitazone (n = 3) than with glibenclamide (n = 9; p = 0.0907). Significantly (p ≤0.05) fewer cases of ALT >1.5 × ULN, aspartate aminotransferase >1.5 × ULN and g-glutamyl transpeptidase >1.5 × ULN were seen with pioglitazone compared with glibenclamide. No case of hepatic dysfunction or hepatic failure was reported in either treatment group; two cases of hepatic cirrhosis with glibenclamide were reported.
Conclusion: This study demonstrates an hepatic safety profile of pioglitazone similar to that of glibenclamide in long-term use in patients with poorly controlled type 2 diabetes.
Trial registration number (clinicaltrials.gov): NCT00494312







Similar content being viewed by others
Notes
1Trial registration number (clinicaltrials.gov): NCT00494312.
References
Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40: 1387–95
Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002; 346: 1221–31
ACTOS (pioglitazone hydrochloride tablets for oral administration) [package insert]. Lincolnshire (IL): Takeda Pharmaceuticals North America, Inc, revised 2004 Aug
Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 2006; 10: 934–45
Diehl AM. Tumor necrosis factor and its potential role in insulin resistance and nonalcoholic fatty liver disease. Clin Liver Dis 2004; 8: 619–38
Uysal KT, Wiesbrock SM, Marino MW, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997; 389: 610–4
Belfort R, Harrison SA, Brown K, et al. A placebocontrolled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006; 355: 2297–307
Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. LIDO Study Group. Gastroenterology 2008; 135: 100–10
Tolman KG, Fonseca V, Tan MH, et al. Narrative review: hepatobiliary disease in type 2 diabetes mellitus. Ann Intern Med 2004; 141: 946–56
Avigan M. Characterization of DILI risk from post marketing reports. FDA/CDER-AASLD-PhRMA Hepatotoxicity Steering Group Meeting, 2006 Jan 25–26, North Bethesda (MD) [online]. Available from URL: http://www.fda.gov/downloads/Drugs/ScienceResearch/ResearchAreas/ucm079518.pdf [Accessed 2009 Jul 2]
Ahmad SR. Adverse drug monitoring at the Food and Drug Administration. J Gen Intern Med 2003; 18: 57–60
Chalasani N, Teal E, Hall SD. Effect of rosiglitazone on serum liver biochemistries in diabetic patients with normal and elevated baseline liver enzymes. Am J Gastroenterol 2005; 100: 1317–21
Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366: 1279–89
Belcher G, Lambert C, Edwards G, et al. Safety and tolerability of pioglitazone, metformin, and gliclazide in the treatment of type 2 diabetes. Diab Res Clin Pract 2005; 70: 53–62
Kasliwal R, Wilton LV, Shakir SAW. Monitoring the safety of pioglitazone: results of a prescription-event monitoring study of 12 772 patients in England. Drug Saf 2008; 31: 839–50
Micronase® (glyburide) [package insert]. Kalamazoo (MI): Pharmacia, revised 2002 Mar
Goodman RC, Dean PJ, Radparvar A, et al. Glyburideinduced hepatitis. Ann Intern Med 1987; 106: 837–9
Van Basten JP, van Hoek B, Zeijen R. Glyburide-induced cholestatic hepatitis and liver failure: case report and review of the literature. Neth J Med 1992; 40: 305–7
Saw D, Pitman E, Maung M, et al. Granulomatous hepatitis associated with glyburide. Dig Dis Sci 1996; 41: 322–5
American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care 2000; 23 Suppl. 1: S27–31
Riley MR, Kastrup EK, eds. Drug facts and comparisons. 54th ed. St. Louis (MO): Wolters Kluwer Company, 2000
World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects [online]. Available from URL: http://www.wma.net/e/policy/b3.htm [Accessed 2009 Jul 13]
Danan G, Benichou C. Causality assessment of adverse reaction to drugs: I. A novel method based on the conclusions of international consensus meetings: application to druginduced liver injuries. J Clin Epidemiol 1993; 46: 1323–30
Agresti A. Categorical data analysis. New York: John Wiley & Sons, Inc., 1990
Mitchell JR, Long MW, Thorgeirsson UP, et al. Acetylation rates and monthly liver function tests during one year of isoniazid preventive therapy. Chest 1975; 68: 181–90
Senior JR. Adaptation to liver injury: tacrine, isoniazid, ethanol, experimental drugs. Hepatotoxicity Steering Group 2006 Meeting, North Bethesda (MD), 2005 Jan 25–26 [online]. Available from URL: http://www.fda.gov/cder/livertox/presentations2006/senior.htm [Accessed 2008 Jul 28]
Food and Drug Administration. Seventy-third meeting of the Endocrinology and Metabolic Drug Advisory Board, 23 April 1999 [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/99/transcpt/3507t2.rtf [Accessed 2009 Jul 13] 800
Zimmerman HJ. Drug-induced liver disease. In: Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 1st ed. New York (NY): Appleton-Century-Crofts, 1978: 351–3
Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf 2006; 15: 241–3
Reuben A. Hy’s law. Hepatology 2004; 39: 574–8
FDA approves pioglitazone to treat type 2 diabetes. Food and Drug Administration, US Department of Health and Human Services, July 16 1999 [online]. Available from URL: http://www.fda.gov/bbs/topics/ANSWERS/ANS00965.html [Accessed 2009 May 1]
Avandia (rosiglitazone maleate) [package insert]. Research Triangle Park (NJ): GlaxoSmithKline, 2007 Aug
Isley WL. Hepatotoxicity of thiazolidinediones. Expert Opin Drug Saf 2003; 2: 581–6
Rajagopalan R, Iyer S, Perez A. Comparison of pioglitazone with other antidiabetic drugs for associated incidence of liver failure: no evidence of increased risk of liver failure with pioglitazone. Diabetes Obes Metab 2005; 7: 161–9
Lebovitz HE, Kreider M, Freed MI. Evaluation of liver function in type 2 diabetic patients during clinical trials: evidence that rosiglitazone does not cause hepatic dysfunction. Diabetes Care 2002; 25: 815–21
Zawadzki JK, Green L, Graham DJ. Thiazolidinedioneassociated observed 15-month post marketing hepatotoxicity. FDA science forum [online]. Available from URL: http://www.accessdata.fda.gov/ScienceForums/forum02/AB-04.htm [Accessed 2009 Jul 13]
Chan KA, Truman A, Gurwitz JH, et al. A cohort study of the incidence of serious acute liver injury in diabetic patients treated with hypoglycemic agents. Arch Intern Med 2003; 163: 728–34
Tolman KC, Chandramouli J. Hepatoxicity of the thiazolidinediones. Clin Liver Dis 2003; 7: 369–79
Kahn SE, Haffner SM, Heise MA, et al., for the ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–43
Waugh J, Keating GM, Plosker GL, et al. Pioglitazone: a review of its use in type 2 diabetes mellitus [published erratum appears in Drugs 2006; 66 (3): 340–1]. Drugs 2006; 66: 85–109
Prueksaritanont T, Vega JM, Zhao J, et al. Interactions between simvastatin and troglitazone or pioglitazone in healthy subjects. J Clin Pharmacol 2001; 41: 573–81
MacDonald JS, Gerson RJ, Kornbrust DJ, et al. Preclinical evaluation of lovastatin. Am J Cardiol 1988; 62 Suppl.: 16–27J
Tolman KG. The liver and lovastatin. Am J Cardiol 2002; 89: 1374–80
Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology 2005; 41: 690–5
Chalasani N, Aljadhey H, Kesterson J, et al. Patients with elevated liver enzymes are not a higher risk for statin hepatotoxicity. Gastroenterology 2004; 128: 1287–92
Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. Am J Med Sci 2005; 329: 62–5
Khorashadi S, Hasson NK, Cheung RC. Incidence of statin hepatotoxicity in patients with hepatitis C. Clin Gastroenterol Hepatol 2006; 4: 902–7
Horlander J, Kwo P, Cummings O. Atorvastatin for the treatment of NASH [abstract]. Gastroenterology 2001; A-544 (2767)
Kiyici M, Gulten M, Gurel S, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol 2003; 17: 713–8
Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis 2004; 174: 193–6
Antonopoulos S, Mikros S, Mylonopoulou M, et al. Rosuvastatin as a novel treatment of non-alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis 2006; 184: 233–4
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009; 52: 17–30
Polonsky WH, Fisher L, Guzman S, et al. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care 2005; 28: 2543–5
Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy: the basis of patient reluctance. Diabetes Care 1997; 20: 292–8
Meece J. Dispelling myths and removing barriers about insulin in type 2 diabetes. Diabetes Educ 2006; 32 Suppl. 1: 9–18S
Davidson CS, Leevy CM, Chamberlayne EC, editors. Guidelines for detection of hepatotoxicity due to drugs and chemicals. Fogerly Conference, 1978. Washington, DC: US Government Printing Office, 1979. NIH publication no. 79–313
Mehendale HM. Liver tissue. Hepatotoxicity Steering Group 2006 Meeting; 2006 Jan 25–26; North Bethesda (MD) [online]. Available from URL: http://www.fda.gov/downloads/Drugs/ScienceResearch/ResearchAreas/ucm079350.pdf [Accessed 2009 Jul 13]
Senior JR. Regulatory perspectives. In: Kaplowitz N, DeLeve LD, editors. Drug-induced liver disease. 2nd ed. New York: Informa Health Care, 2007
Acknowledgements
The authors wish to acknowledge the writing, editing and manuscript formatting assistance of Craig Lyon of Lyonize, Inc., whose services were funded by Takeda Pharmaceuticals North America, Inc. Keith Tolman has been a consultant to and is on the Drug Safety Monitoring Board of Takeda Pharmaceuticals, and has received honoraria from Takeda Pharmaceuticals and Lilly. James Freston has acted as a consultant to Takeda Pharmaceuticals intermittently since 1998, and to GlaxoSmithKline since 2006. Stuart Kupfer and Alfonso Perez are employees of Takeda Global Research and Development. The study was sponsored and fully funded by Takeda Global Research and Development Centre, Inc., Deerfield, IL, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tolman, K.G., Freston, J.W., Kupfer, S. et al. Liver Safety in Patients with Type 2 Diabetes Treated with Pioglitazone. Drug-Safety 32, 787–800 (2009). https://doi.org/10.2165/11316510-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11316510-000000000-00000