Abstract
Background: Lennox-Gastaut syndrome (LGS) is a catastrophic childhood form of epilepsy. The syndrome is characterized bymental impairment, frequent seizures of multiple types that are particularly resistant to treatment, and high rates of seizure-related injury. With the introduction of newer, but more costly, antiepileptic drugs (AEDs), it is important that decision makers are able to assess their value in the management of this rare and difficult-to-treat condition.
Objective: To evaluate the cost effectiveness, from the UK NHS perspective, of rufinamide in patients with LGS.
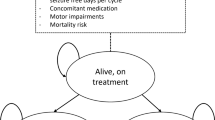
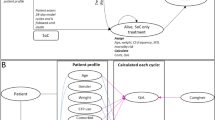
Methods: An individual patient-simulation model was developed to estimate the total treatment-related costs and clinical benefits of rufinamide compared with topiramate and lamotrigine over a 3-year time horizon. The model examines the treatment scenarios of adding rufinamide, lamotrigine or topiramate to older AEDs (standard therapy), or standard therapy alone within a primary-care or community setting.
Three placebo-controlled clinical trials of adjunctive AED treatment for children with LGS were analysed. There are no head-to-head comparator studies. Between 98 and 139 patients were randomized in each study and the mean age in each study was 10, 11 and 14 years. A mixed-treatment comparison using a random-effectsmodel was carried out on the number of patients in each response category, using the placebo arms of the respective trials. The primary outcome measure was the percentage of successfully treated patients, defined as >50% reduction in the frequency of total seizures and drop attacks. The hypothesis being tested was formulated after data collection.
Costs (£, year 2006/07 values) of patient monitoring, switching treatments, hospitalization due to seizure, treatment of adverse effects, and personal and social services were included in the analysis. Results of 10 000 Monte Carlo simulations were bootstrapped to conduct probabilistic sensitivity analysis.
Results: Over 3 years, adjunctive rufinamide resulted in higher total costs than topiramate and lamotrigine; however, with more patients being treated successfully, this leads to acceptable incremental cost-effectiveness ratios. If society is prepared to pay at least d250 for a 1% increase in the number of successfully treated LGS patients, in terms of a 50% reduction in the frequency of drop attacks, the probability of the treatment with rufinamide being cost effective is >80%.
Conclusion: This cost-effectiveness analysis suggests that rufinamide results in more LGS patients being treated successfully at a reasonable cost from a UK NHS perspective.






Similar content being viewed by others
References
Trevathan E, Murphy CC, Yeargin-Allsopp M. Prevalence and descriptive epidemiology of Lennox-Gastaut syndrome among Atlanta children. Epilepsia 1997 Dec; 38 (12): 1283–8
Cheng-Hakimian A, Anderson GD, Miller JW. Rufinamide: pharmacology, clinical trials, and role in clinical practice. Int J Clin Pract 2006 Nov; 60 (11): 1497–501
Camfield P, Camfield C. Epileptic syndromes in childhood: clinical features, outcomes, and treatment. Epilepsia 2002; 43 Suppl. 3: 27–32
Roger J, Dravet C, Bureau M. The Lennox-Gastaut syndrome. Cleve Clin J Med 1989; 56 Suppl. Pt 2: S172–80
French JA. The role of new antiepileptic drugs. Am J Manag Care 2001 Jul; 7 (7 Suppl.): S209–14
Wheless JW, Clarke DF, Arzimanoglou A, et al. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Disord 2007 Dec; 9 (4): 353–412
Arroyo S, de la Morena A. Life-threatening adverse events of antiepileptic drugs. Epilepsy Res 2001 Nov; 47 (1-2): 155–74
Hancock E, Cross H. Treatment of Lennox-Gastaut syndrome. Cochrane Database Syst Rev 2003; (3): CD00377
Thompson PJ, Baxendale SA, Duncan JS, et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry 2000 Nov; 69 (5): 636–41
McElroy SL, Hudson JI, Capece JA, et al. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry 2007 May; 61 (9): 1039–48
Glauser TA, Kluger G, Sachdeo R, et al. Rufinamide for generalized seizures associated with Lennox-Gastaut syndrome. Neurology 2008; 71 (21): 1950–8
McLean MJ, Schmutz M, Pozza MF, et al. Effects of rufiniamide on sodium-dependent action potential firing and sodium currents of rodent central neurons [abstract]. Epilepsia 2005; 46 Suppl. 6: 135
Arroyo S. Rufinamide. Neurotherapeutics 2007 Jan; 4 (1): 155–62
Yagi K. Evolution of Lennox-Gastaut syndrome: a longterm longitudinal study. Epilepsia 1996; 37 Suppl. 3: 48–51
Data on file, Eisai, 2005
Motte J, Trevathan E, Arvidsson JF, et al., on behalf of the Lamictal Lennox-Gastaut Study Group. Lamotrigine for generalized seizures associated with the Lennox-Gastaut syndrome. N Engl J Med 1997 Dec; 337 (25): 1807–12
Sachdeo RC, Glauser TA, Ritter F, et al., on behalf of the Topiramate YL Study Group. A double-blind, randomized trial of topiramate in Lennox-Gastaut syndrome. Neurology 1999 Jun; 52 (9): 1882–7
Lhatoo SD, Johnson AL, Goodridge DM, et al. Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long-term, prospective, population-based cohort. Ann Neurol 2001 Mar; 49 (3): 336–44
NHS Reference Costs 2005/2006 [online]. Available from URL: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_062884 [Accessed 2009 Oct 30]
Personal Social Services Research Unit (PSSRU). Unit costs of health and social care, 2006 [online]. Available from URL: http://www.pssru.ac.uk/pdf/uc/uc2006/uc2006.pdf [Accessed 2009 Oct 30]
British national formulary no. 53, 2007 [online]. Available from URL: http://www.bnf.org/bnf/ [Accessed 2007 Sep]
GMB survey. London: Britain’s General Union, 2006 [online]. Available from URL: http://www.gmb.org.uk/Templates/PressItems.asp?NodeID=93132 [Accessed 2009 Oct 30]
National Institute forHealth and Clinical Excellence. Updated guide to the methods of technology appraisal. London: NICE, 2008 [online]. Available from URL: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf [Accessed 2009 Oct 30]
Connock M, Frew E, Evans BW, et al. The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy: a systematic review. Health Technol Assess 2006 Mar; 10 (7): iii, ix–118
Glauser TA, Levisohn PM, Ritter F, et al., on behalf of the Topiramate YL Study Group. Topiramate in Lennox-Gastaut syndrome: open-label treatment of patients completing a randomized controlled trial. Epilepsia 2000; 41 Suppl. 1: S86–90
Schachter S, Sommerville KW, Ryan JE. A cost-effectiveness analysis of tiagabine, phenytoin, and carbamazepine adjunctive therapy for patients with complex partial seizures [abstract]. Neurology 1999; 52 Suppl. 6: A143
Wilby J, Kainth A, Hawkins N, et al. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol Assess 2005 Apr; 9 (15): 1–157, iii-iv
Berto P. Quality of life in patients with epilepsy and impact of treatments. Pharmacoeconomics 2002; 20 (15): 1039–59
Alanis-Guevara I, Pena E, Corona T, et al. Sleep disturbances, socioeconomic status, and seizure control as main predictors of quality of life in epilepsy. Epilepsy Behav 2005 Nov; 7 (3): 481–5
McLaughlin DP, Pachana NA, McFarland K. Stigma, frequency and quality of life: the impact of epilepsy in late adulthood. Seizure 2008 Apr; 17 (3): 281–7
Wheless JW. Intractable epilepsy: a survey of patients and caregivers. Epilepsy Behav 2006 Jun; 8 (4): 756–64
Baker GA, Jacoby A, Buck D, et al. Quality of life of people with epilepsy: a European study. Epilepsia 1997 Mar; 38 (3): 353–62
Devinsky O, Westbrook L, Cramer J, et al. Risk factors for poor health-related quality of life in adolescents with epilepsy. Epilepsia 1999 Dec; 40 (12): 1715–20
Derry PA, Wiebe S. Psychological adjustment to success and to failure following epilepsy surgery. Can J Neurol Sci 2000 May; 27 Suppl. 1: S116–20
Glenny AM, Altman DG, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005 Jul; 9 (26): 1–134, iii-iv
Song F, Altman DG, Glenny AM, et al. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003 Mar; 326 (7387): 472
Sutton A, Ades AE, Cooper N, et al. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics 2008; 26 (9): 753–67
Bombardier C, Maetzel A. Pharmacoeconomic evaluation of new treatments: efficacy versus effectiveness studies? Ann Rheum Dis 1999 Nov; 58 Suppl. 1: I82–5
Majoie HJ, Berfelo MW, Aldenkamp AP, et al. Vagus nerve stimulation in children with therapy-resistant epilepsy diagnosed as Lennox-Gastaut syndrome: clinical results, neuropsychological effects, and cost-effectiveness. J Clin Neurophysiol 2001 Sep; 18 (5): 419–28
Selai CE, Smith K, Trimble MR. Adjunctive therapy in epilepsy: a cost-effectiveness comparison of two AEDs. Seizure 1999 Feb; 8 (1): 8–13
Acknowledgements
Ágnes Benedict is an employee of UBC. UBC received payment for this and other consultancy work from Eisai Ltd. UBC Health Care Analytics is an independent health outcomes consultancy that performs consultancy work for a number of pharmaceutical companies. Grant Maclaine and Lara Verdian are employees of Eisai Europe Limited.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.2165/11598620-000000000-00000.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Benedict, Á., Verdian, L. & Maclaine, G. The Cost Effectiveness of Rufinamide in the Treatment of Lennox-Gastaut Syndrome in the UK. Pharmacoeconomics 28, 185–199 (2010). https://doi.org/10.2165/11313640-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11313640-000000000-00000