Abstract
Etravirine (Intelence®) is an orally administered next-generation non-nucleoside reverse transcriptase inhibitor (NNRTI). It is approved for the treatment of HIV-1 infection in treatment-experienced adult patients who have evidence of viral replication and are harbouring HIV-1 strains resistant to other antiretroviral (ARV) agents. In the US, etravirine must be used in combination with other ARV agents; in the EU, it must be used in combination with other ARV agents that include a boosted HIV-1 protease inhibitor.
Etravirine shows good activity in vitro against most wild-type strains of HIV-1, as well as against several strains resistant to available NNRTIs. Furthermore, etravirine appears to present a higher barrier than first-generation NNRTIs against the development of drug resistance. Whereas the presence of a single mutation is sufficient to affect the virological response to efavirenz or nevirapine, the resistance profile of etravirine is more complex and a prediction of virological response may be calculated using a weighted genotypic score. Importantly, the most prevalent NNRTI-associated mutation, K103N, alone does not affect the etravirine response.
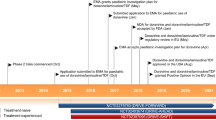
In two identically designed randomized clinical trials, the addition of etravirine to an optimized background therapy (OBT) regimen improved virological responses to a greater extent than placebo plus OBT following 24 weeks’ treatment in highly treatment-experienced adult patients with HIV-1 infection who had evidence of viral replication (HIV-1 RNA levels of >5000 copies/mL at baseline). Furthermore, pre-planned pooled analyses of the trials at 48 and 96 weeks showed that etravirine plus OBT provided durable virological suppression. Consistently higher virological response rates were observed for recipients of etravirine plus OBT than placebo plus OBT in a pre-specified subgroup analysis of baseline viral loads, CD4+ cell counts, HIV-1 subtype or the composition of background ARV therapy. Greater improvements from baseline in immunological outcomes were also observed for recipients of etravirine plus OBT compared with those receiving placebo plus OBT over the 96-week treatment period of the trials.
When used as part of an OBT regimen in trials of up to 96 weeks duration, etravirine was well tolerated with an overall tolerabilty profile similar to that of placebo. The only treatment-emergent adverse event that occurred with a higher frequency for recipients of etravirine compared with placebo plus OBT was rash.
In highly treatment-experienced patients with HIV-1 infection and evidence of viral replication, the addition of etravirine to an OBT regimen provides an effective and well tolerated treatment that leads to improvements in both virological and immunological outcomes.








Similar content being viewed by others
References
Shet A, Berry L, Mohri H, et al. Tracking the prevalence of transmitted antiretroviral drug-resistant HIV-1: a decade of experience. J Acquir Immune Defic Syndr 2006 Apr 1; 41(4): 439–46
Chaix ML, Desquilbet L, Descamps D, et al. Response to HAART in French patients with resistant HIV-1 treated at primary infection: ANRS Resistance Network. Antivir Ther 2007; 12(8): 1305–10
Hogg RS, Bangsberg DR, Lima VD, et al. Emergence of drug resistance is associated with an increased risk of death among patients first starting HAART. PLoS Med 2006 Sep;3(9): 1570–8
Losina E, Yazdanpanah Y, Deuffic-Burban S, et al. The independent effect of highly active antiretroviral therapy on severe opportunistic disease incidence and mortality in HIV-infected adults in Cote d’Ivoire. Antivir Ther 2007; 12(4): 543–51
Vercauteren J, Deforche K, Theys K, et al. The incidence of multidrug and full class resistance in HIV-1 infected patients is decreasing over time (2001–2006) in Portugal. Retrovirology 2008; 5: 12
SPREAD Programme. Transmission of drug-resistant HIV-1 in Europe remains limited to single classes. AIDS 2008; 22(5): 625–30
DHHS panel on antiretroviral guidelines for adults and adolescents: a working group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents [online]. Available from URL: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [Accessed 2012 Mar 16]
Asboe D, Aitken C, Boffito M, et al. British HIV Associa-tion guidelines for the routine investigation and monitoring of adult HIV-1-infected individuals 2011. HIV Med 2012 Jan; 13(1): 1–44
Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 2010 Jul 21; 304(3): 321–33
European AIDS Clinical Society (EACS). Guidelines for the treatment of HIV infected adults in Europe [online]. Available from URL: http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/eacsguidelines-v6_english.pdf [Accessed 2012 Jan 16]
Pedersen OS, Pedersen EB. Non-nucleoside reverse transcriptase inhibitors: the NNRTI boom. Antivir Chem Chemother 1999 Nov; 10(6): 285–314
Gardner EM, Hullsiek KH, Telzak EE, et al. Antiretroviral medication adherence and class-specific resistance in a large prospective clinical trial. AIDS 2010 Jan 28; 24(3): 395–403
Richman DD, Morton SC, Wrin T, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS 2004; 18: 1393–401
Grant RM, Hecht FM, Warmerdam M, et al. Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA 2002 Jul 10; 288(2): 181–8
Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis 2008 Jul 15; 47(2): 266–85
Deeks ED, Keating GM. Etravirine. Drugs 2008; 68(16): 2357–72
Tibotec I. Intelence (etravirine) tablets: US prescribing information [online]. Available from URL: http://www.intelence-info.com/sites/default/files/pdf/INTELENCE_Booklet_Package_Insert_hcp.pdf [Accessed 2012 Feb 10]
European Medicines Agency. Intelence 100 mg tablets: sum-mary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000900/WC500034180.pdf [Accessed 2012 Feb 10]
Figueiredo A, Moore KL, Mak J, et al. Potent nonnucleo-side reverse transcriptase inhibitors target HIV-1 Gag-Pol. PLoS Pathog 2006 Nov; 2(11): e119
Das K, Clark AD, Lewi PJ, et al. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J Med Chem 2004 May 6; 47(10): 2550–60
Andries K, Azijn H, Thielemans T, et al. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob Agents Chemother 2004 Dec; 48(12): 4680–6
Vingerhoets J, Azijn H, Fransen E, et al. TMC125 displays a high genetic barrier to the development of resistance: evidence from in vitro selection experiments. J Virol 2005 Oct; 79(20): 12773–82
Vingerhoets J, Tambuyzer L, Azijn H, et al. Resistance profile of etravirine: combined analysis of baseline geno-typic and phenotypic data from the randomized, controlled phase III clinical studies. AIDS 2010; 24(4): 503–14
Tambuyzer L, Vingerhoets J, Azijn H, et al. Characterization of genotypic and phenotypic changes in HIV-1-infected patients with virologic failure on an etravirine-containing regimen in the DUET-1 and DUET-2 clinical studies. AIDS Res Hum Retroviruses 2010; 26(11): 1197–205
Shortle D, Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci USA 1978 May; 75(5): 2170–4
Peeters M, Vingerhoets J, Tambuyzer L, et al. Etravirine limits the emergence of darunavir and other protease inhibitor resistance-associated mutations in the DUET trials. AIDS 2010; 24(6): 921–4
Peeters M, Janssen K, Kakuda TN, et al. Etravirine has no effect on QT and corrected QT interval in HIV-negative volunteers. Ann Pharmacother 2008 Jun; 42(6): 757–65
Scholler-Gyure M, Kakuda TN, Smedt GD, et al. Effects of hepatic impairment on the steady-state pharmacokinetics of etravirine 200 mg BID: an open-label, multiple-dose, controlled phase I study in adults. Clin Ther 2010; 32(2): 328–37
Scholler-Gyure M, Boffito M, Pozniak AL, et al. Effects of different meal compositions and fasted state on the oral bioavailability of etravirine. Pharmacotherapy 2008; 28(10): 1215–22
Scholler-Gyure M, Kakuda TN, Raoof A, et al. Clinical pharmacokinetics and pharmacodynamics of etravirine. Clin Pharmacokinet 2009; 48(9): 561–74
Kakuda TN, Scholler-Gyure M, Workman C, et al. Single-and multiple-dose pharmacokinetics of etravirine administered as two different formulations in HIV-1-infected patients. Antiviral Ther 2008; 13(5): 655–61
Patterson K, Jennings S, Falcon R, et al. Darunavir, ritonavir, and etravirine pharmacokinetics in the cervicovaginal fluid and blood plasma of HIV-infected women. Antimicrob Agents Chemother 2011; 55(3): 1120–2
Kakuda TN, Scholler-Gyure M, Hoetelmans RMW. Clinical perspective on antiretroviral drug-drug interactions with the non-nucleoside reverse transcriptase inhibitor etravirine. Antiviral Ther 2010; 15(6): 817–29
DeJesus E, Lalezari JP, Osiyemi OO, et al. Pharmacokinetics of once-daily etravirine without and with once-daily darunavir/ritonavir in antiretroviral-naive HIV type-1-in-fected adults. Antiviral Ther 2010; 15(5): 711–20
ter Heine R, Mulder JW, Van GECM, et al. Intracellular and plasma steady-state pharmacokinetics of raltegravir, darunavir, etravirine and ritonavir in heavily pre-treated HIV-infected patients. Br J Clin Pharmacol 2010; 69(5): 475–83
Kakuda TN, Wade JR, Snoeck E, et al. Pharmacokinetics and pharmacodynamics of the non-nucleoside reverse-transcriptase inhibitor etravirine in treatment-experienced HIV-1-infected patients. Clin Pharmacol Ther 2010; 88(5): 695–703
Izurieta P, Kakuda TN, Feys C, et al. Safety and pharmacokinetics of etravirine in pregnant HIV-1-infected women. HIV Med 2011; 12(4): 257–8
Konigs C, Feiterna-Sperling C, Esposito S, et al. Pharmacokinetics and short-term safety and tolerability of etravirine in treatment-experienced HIV-1-infected children and adolescents. AIDS 2012 Feb 20; 26(4): 447–55
Kakuda TN, Scholler-Gyure M, Hoetelmans RMW. Pharmacokinetic interactions between etravirine and nonantiretroviral drugs. Clin Pharmacokinet 2011; 50(1): 25–39
University of Liverpool. Drug interaction charts [online]. Available from URL: http://www.hiv-druginteractions.org/Interactions.aspx [Accessed 2012 Feb 20]
University of California San Francisco. Database of antiretroviral drug interactions [online]. Available from URL: http://arv.ucsf.edu/insite?page=ar-00-02 [Accessed 2012 Mar 12]
Song I, Borland J, Min S, et al. Effects of etravirine alone and with ritonavir-boosted protease inhibitors on the pharmacokinetics of dolutegravir. Antimicrob Agents Chemother 2011 Jul; 55(7): 3517–21
Ramanathan S, Kakuda TN, Mack R, et al. Pharmacokinetics of elvitegravir and etravirine following coadministration of ritonavir-boosted elvitegravir and etravirine. Antivir Ther 2008; 13(8): 1011–7
Madruga JV, Cahn P, Grinsztejn B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007 Jul 7; 370(9581): 29–38
Lazzarin A, Campbell T, Clotet B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007 Jul 7; 370(9581): 39–48
Katlama C, Haubrich R, Lalezari J, et al. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS 2009; 23(17): 2289–300
Katlama C, Clotet B, Mills A, et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antiviral Ther 2010; 15(7): 1045–52
Yazdanpanah Y, Fagard C, Descamps D, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis 2009; 49(9): 1441–9
Fagard C, Colin C, Charpentier C, et al. Long-term efficacy and safety of raltegravir, etravirine, and darunavir/ ritonavir in treatment-experienced patients: week 96 results from the ANRS 139 TRIO trial. J Acquir Immune Defic Syndr 2012 Apr 15; 59(5): 489–93
Towner W, Lalezari J, Sension MG, et al. Efficacy, safety, and tolerability of etravirine with and without darunavir/ ritonavir or raltegravir in treatment-experienced patients: analysis of the etravirine early access program in the United States. J Acquired Immune Defic Syndr 2010; 53(5): 614–8
Santos JR, Llibre JM, Domingo P, et al. Short communication: high effectiveness of etravirine in routine clinical practice in treatment-experienced HIV type 1-infected patients. AIDS Res Hum Retroviruses 2011; 27(7): 713–7
Cella D, Gilet H, Viala-Danten M, et al. Effects of etravirine versus placebo on health-related quality of life in treatment-experienced HIV patients as measured by the functional assessment of human immunodeficiency virus infection (FAHI) questionnaire in the DUET trials. HIV Clin Trials 2010; 11(1): 18–27
Cahn P, Haubrich R, Katlama C, et al. The impact of baseline characteristics on virologic response to etravirine: 48-week pooled analysis of DUET-1 and DUET-2. HIV Ther 2010; 4(5): 605–10
Clumeck N, Cahn P, Molina J-M, et al. Virological response with fully active etravirine: pooled results from the DUET-1 and DUET-2 trials. Int J STD AIDS 2010; 21(11): 738–40
Trottier B, Di PG, Madruga J, et al. Impact of the background regimen on virologic response to etravirine: pooled 48-week analysis of DUET-1 and-2. HIV Clin Trials 2010; 11(4): 175–85
Anderson D, DeMasi R, DeLaitsch L, et al. Week 96 outcomes of patients with less versus more treatment experience receiving etravirine in the DUET trials [abstract no. TPOI-5]. 4th Annual American Conference for the Treatment of HIV (ACTHIV); 2011 Apr 7–9; Denver (CO)
Clotet B, Clumeck N, Katlama C, et al. Safety of etravirine in HIV-1/hepatitis B and/or C virus co-infected patients: pooled 96 week results from the phase III DUET trials. J Antimicrob Chemother 2010; 65(11): 2450–4
Nelson M, Stellbrink H-J, Podzamczer D, et al. A comparison of neuropsychiatric adverse events during 12 weeks of treatment with etravirine and efavirenz in a treatmentnaive, HIV-1-infected population. AIDS 2011; 25(3): 335–40
Gazzard B, Duvivier C, Zagler C, et al. Phase 2 double-blind, randomized trial of etravirine versus efavirenz in treatment-naive patients: 48-week results. AIDS 2011; 25(18): 2249–58
Fatkenheuer G, Duvivier C, Rieger A, et al. Lipid profiles for etravirine versus efavirenz in treatment-naive patients in the randomized, double-blind SENSE trial. J Antimicrob Chemother 2012; 67(3): 685–90
Mauskopf J, Brogan AJ, Talbird SE, et al. Cost-effectiveness of combination therapy with etravirine in treatment-experienced adults with HIV-1 infection. AIDS 2012 Jan 28; 26(3): 355–64
National Guideline Clearinghouse. Antiretroviral therapy [online]. Available from URL: http://www.guideline.gov/content.aspx?id=24034 [Accessed 2012 Feb 1]
Fletcher CV. Translating efficacy into effectiveness in antiretroviral therapy: beyond the pill count. Drugs 2007; 67(14): 1969–79
Waters L, John L, Nelson M. Non-nucleoside reverse transcriptase inhibitors: a review. Int J Clin Pract 2007 Jan; 61(1): 105–18
Li JZ, Paredes R, Ribaudo HJ, et al. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based anti-retroviral treatment failure: a systematic review and pooled analysis. JAMA 2011 Apr 6; 305(13): 1327–35
FDA. Important information about sustiva (efavirenz) and pregnancy [online]. Available from URL: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm124885.htm [Accessed 2012 Feb 21]
Delaugerre C, Rohban R, Simon A, et al. Resistance profile and cross-resistance of HIV-1 among patients failing a non-nucleoside reverse transcriptase inhibitor-containing regimen. J Med Virol 2001 Nov; 65(3): 445–8
Stellbrink H-J. Etravirine (TMC-125): the evidence for its place in the treatment of HIV-1 infection. Core Evid 2009; 4: 149–58
Tambuyzer L, Nijs S, Daems B, et al. Effect of mutations at position E138 in HIV-1 reverse transcriptase on phenotypic susceptibility and virologic response to etravirine. J Acquir Immune Defic Syndr 2011; 58(1): 18–22
Das K, Bauman JD, Clark AD, et al. High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. Proc Natl Acad Sci U S A 2008 Feb 5; 105(5): 1466–71
Fulco PP, McNicholl IR. Etravirine and rilpivirine: nonnucleoside reverse transcriptase inhibitors with activity against human immunodeficiency virus type 1 strains resistant to previous nonnucleoside agents. Pharmacotherapy 2009; 29(3): 281–94
Sanford M. Rilpivirine. Drugs 2012; 72(4): 525–41
Dahri K, Ensom MH. Efavirenz and nevirapine in HIV-1 infection: is there a role for clinical pharmacokinetic monitoring? Clin Pharmacokinet 2007; 46(2): 109–32
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: C. Katalama, Université Pierrre et Marie Curie, Paris, France; R. MacArthur, Division of Infectious Diseases, Wayne State University, Detroit, MI, USA; I. McNicholl, Department of Medicine, University of California, San Francisco, CA, USA; M. Nelson, Chelsea and Westminster Hospital, London, UK; R. ter Heine, Department of Pharmacy and Pharmacology, Slotervaart Hospital, Amsterdam, the Netherlands.
Data Selection Sources: Medical literature (including published and unpublished data) on ‘etravirine’ was identified by searching databases (including MEDLINE and EMBASE) for articles published since 1996, bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the company developing the drug. Search strategy: MEDLINE and EMBASE search terms were ‘etravirine’ and (‘HIV infection’ or ‘human immunodeficiency virus infection’). Searches were last updated on 26 March 2012. Selection:: Studies in patients with HIV infection who received etravirine. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included. Index terms: Etravirine, HIV-1 infection, antiretroviral, non-nucleoside reverse transcriptase inhibitor, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Croxtall, J.D. Etravirine. Drugs 72, 847–869 (2012). https://doi.org/10.2165/11209110-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11209110-000000000-00000