Abstract
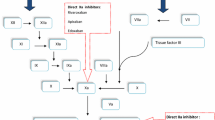
Apixaban (Eliquis™) is an orally active and selective direct inhibitor of factor Xa indicated for twice-daily use in the EU for the prevention of venous thromboembolism (VTE) in adults who have had knee or hip replacement surgery. In this article, the pharmacological, clinical efficacy and tolerability data relevant to the use of apixaban in this indication are reviewed.
Oral apixaban is a generally effective and well tolerated thromboprophylactic agent for use after major orthopaedic surgery. In the large, randomized, double-blind, phase III, noninferiority trials known as ADVANCE-2 and -3, apixaban 2.5 mg twice daily initiated after surgery was generally more effective in preventing VTE in patients undergoing knee or hip replacement surgery than subcutaneous enoxaparin sodium initiated before surgery at the EU recommended dosage of 40 mg once daily, with apixaban conferring this benefit without significantly increasing the risk of bleeding. However, when the same apixaban regimen was compared with the US recommended dosage regimen of subcutaneous enoxaparin sodium (30 mg twice daily, initiated after surgery) in patients undergoing knee replacement surgery in the similarly designed ADVANCE-1 trial, the thromboprophylactic efficacy of apixaban did not meet primary endpoint non-inferiority criteria, although apixaban was associated with fewer major or clinically relevant nonmajor bleeds (composite endpoint) than this enoxaparin sodium regimen. Additional comparative efficacy and tolerability data are required to definitively position apixaban with respect to other anticoagulants, including rivaroxaban and dabigatran etexilate. In the meantime, currently available clinical data indicate that apixaban is an emerging option for the prevention of VTE in patients undergoing knee or hip replacement surgery.






Similar content being viewed by others
References
Previtali E, Bucciarelli P, Passamonti SM, et al. Risk factors for venous and arterial thrombosis. Blood Transfus 2011 Apr; 9 (2): 120–38
Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost 2005 Aug; 3 (8): 1611–7
Deitelzweig SB, McKean SC, Amin AN, et al. Prevention of venous thromboembolism in the orthopedic surgery patient. Cleve Clin J Med 2008 Apr; 75 Suppl. 3: S27–36
Heit JA. Estimating the incidence of symptomatic post-operative venous thromboembolism: the importance of perspective. JAMA 2012 Jan 18; 307 (3): 306–7
Warwick D, Friedman RJ, Agnelli G, et al. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the Global Orthopaedic Registry. J Bone Joint Surg Br 2007 Jun; 89-B (6): 799–807
Kearon C. Natural history of venous thromboembolism. Circulation 2003 Jun 17; 107 (23 Suppl. 1): I22–30
Imberti D, Prisco D. Oral factor Xa inhibitors for venous thromboembolism prevention in major orthopedic surgery: a review. Pathophysiol Haemost Thromb 2008; 36 (5): 217–26
Schulman S, Majeed A. A benefit-risk assessment of dabigatran in the prevention of venous thromboembolism in orthopaedic surgery. Drug Saf 2011; 34 (6): 449–63
Bauer KA. Recent progress in anticoagulant therapy: oral direct inhibitors of thrombin and factor Xa. J Thromb Haemost 2011 Jul; 9 Suppl. 1: 12–9
Nutescu EA, Shapiro NL, Chevalier A, et al. A pharmacologic overview of current and emerging anticoagulants. Cleve Clin J Med 2005 Apr; 72 Suppl. 1: S2–6
Eikelboom JW, Zelenkofske SL, Rusconi CP. Coagulation factor IXa as a target for treatment and prophylaxis of venous thromboembolism. Arterioscler Thromb Vasc Biol 2010 Mar; 30 (3): 382–7
Prom R, Spinler SA. The role of apixaban for venous and arterial thromboembolic disease. Ann Pharmacother 2011 Oct; 45 (10): 1262–83
Harenberg J, Wehling M. Current and future prospects for anticoagulant therapy: inhibitors of factor Xa and factor IIa. Semin Thromb Hemost 2008 Feb; 34 (1): 39–57
Wang L, Raghavan N, He K, et al. Sulfation of O-demethyl apixaban: enzyme identification and species comparison. Drug Met Dispos 2009 Apr; 37 (4): 802–8
Wong PC, Jiang X. Apixaban, a direct factor Xa inhibitor, inhibits tissue-factor induced human platelet aggregation in vitro: comparison with direct inhibitors of factor VIIa, XIa and thrombin. Thromb Haemost 2010 Aug; 104 (2): 302–10
Jiang X, Crain EJ, Luettgen JM, et al. Apixaban, an oral direct factor Xa inhibitor, inhibits human clot-bound factor Xa activity in vitro [letter]. Thromb Haemost 2009 Apr; 101 (4): 780–2
Luettgen JM, Knabb RM, He K, et al. Apixaban inhibition of factor Xa: microscopic rate constants and inhibition mechanism in purified protein systems and in human plasma. J Enzyme Inhib Med Chem 2011 Aug; 26 (4): 514–26
Pinto DJP, Orwat MJ, Koch S, et al. Discovery of 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide (apixaban, BMS-562247), a highly potent, selective, efficacious, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem 2007 Nov 1; 50 (22): 5339–56
Schumacher WA, Bostwick JS, Stewart AB, et al. Effect of the direct factor Xa inhibitor apixaban in rat models of thrombosis and hemostasis. J Cardiovasc Pharmacol 2010 Jun; 55 (6): 609–16
Wong PC, Crain EJ, Watson CA, et al. Favorable therapeutic index of the direct factor Xa inhibitors, apixaban and rivaroxaban, compared with the thrombin inhibitor dabigatran in rabbits. J Thromb Haemost 2009 Aug; 7 (8): 1313–20
Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, anti-thrombotic and antihemostatic studies. J Thromb Haemost 2008 May; 6 (5): 820–9
Wong PC, Watson CA, Crain EJ. Arterial antithrombotic and bleeding time effects of apixaban, a direct factor Xa inhibitor, in combination with antiplatelet therapy in rabbits. J Thromb Haemost 2008 Oct; 6 (10): 1736–41
Wong P, Watson C, Knabb R, et al. The combination of apixaban, a direct factor Xa inhibitor, with heparin or enoxaparin in rabbits elicits additive antithrombotic effects, with low bleeding [abstract no. 933]. Eur Heart J 2008 Sep; 29 Suppl. 1: 133–4
Becker RC, Alexander JH, Newby LK, et al. Effect of apixaban, an oral and direct factor Xa inhibitor, on coagulation activity biomarkers following acute coronary syndrome. Thromb Haemost 2010 Nov; 104 (5): 976–83
Barrett YC, Wang Z, Frost C, et al. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010 Dec; 104 (6): 1263–71
Barrett YC, Wang J, Knabb R, et al. Apixaban decreases coagulation activity in patients with acute deep-vein thrombosis. Thromb Haemost 2011 Jan; 105 (1): 181–9
Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med 2011 Aug 25; 365 (8): 699–708
Frost C, Song Y, Barrett YC, et al. Direct comparison of the pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban [abstract no. P-WE-159]. J Thromb Haemost 2011 Jul; 9 Suppl. 2: 569–70
Bristol-Myers Squibb/Pfizer EEIG. Eliquis 2.5 mg film-coated tablets: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf [Accessed 2012 May 29]
European Medicines Agency. Assessment report for Eliquis [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002148/WC500107726.pdf [Accessed 2012 May 29]
Wong PC, Pinto DJP, Zhang D. Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis 2011 May; 31 (4): 478–92
Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet 2009; 48 (1): 1–22
Frost CE, Nepal S, Barrett Y, et al. Effects of age and gender on the single-dose pharmacokinetics (PK) and pharmacodynamics (PD) of apixaban [abstract no. PP-MO-407]. J Thromb Haemost 2009 Jul; 7 Suppl. 2: 455
Upreti VV, Wang J, Barrett Y, et al. Effect of body weight on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor, in healthy subjects [abstract no. 16]. J Clin Pharmacol 2010; 50: 1060
Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009 Jan; 37 (1): 74–81
Frost C, Yu Z, Moore K, et al. Apixaban, an oral direct factor Xa inhibitor: multiple-dose safety, pharmacokinetics, and pharmacodynamics in healthy subjects [abstract no. P-M-664]. J Thromb Haemost 2007 Aug; 5 Suppl. 2
Wang L, Zhang D, Raghavan N, et al. In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab Dispos 2010 Mar; 38 (3): 448–58
Zhang D, He K, Raghavan N, et al. Comparative metabolism of 14C-labeled apixaban in mice, rats, rabbits, dogs, and humans. Drug Metab Dispos 2009 Aug; 37 (8): 1738–48
Lassen MR, Davidson BL, Gallus A, et al. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost 2007 Dec; 5 (12): 2368–75
Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 2009 Aug 6; 361 (6): 594–604
Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 2010 Mar 6; 375 (9717): 807–15
Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010 Dec 23; 363 (26): 2487–98
Raskob GE, Gallus AS, Pineo GF, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE-2 and ADVANCE-3 trials. J Bone Joint Surg Br 2012 Feb; 94-B (2): 257–64
Pineo GF, Gallus A, Raskob G, et al. Apixaban vs. enoxaparin after knee or hip surgery: efficacy and safety in key clinical subgroups [abstract no. O-TH-031]. J Thromb Haemost 2011; 9 Suppl. 2: 730–1
Huang J, Cao Y, Liao C, et al. Apixaban versus enoxaparin in patients with total knee arthroplasty: a meta-analysis of randomised trials. Thromb Haemost 2011 Feb 1; 105 (2): 245–53
Department of Health. Report of the independent expert working group on the prevention of venous thromboembolism in hospitalised patients [online]. Available from URL: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_073950.pdf [Accessed 2012 Jan 13]
Baser O. Prevalence and economic burden of venous thromboembolism after total hip arthroplasty or total knee arthroplasty. Am J Manag Care 2011 Feb; 17 (1 Suppl.): S6–8
National Institute for Health and Clinical Excellence. Venous thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital [online]. Available from URL: http://www.nice.org.uk/nicemedia/live/12695/47920/47920.pdf [Accessed 2011 Dec 23]
American Academy of Orthopaedic Surgeons. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty: evidence-based guideline and evidence report [online]. Available from URL: http://www.aaos.org/Research/guidelines/VTE/VTE_full_guideline.pdf [Accessed 2011 Dec 23]
Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012 Feb; 141 (2 Suppl.): e278S–325S
Abad Rico JI, Llau Pitarch JV, Páramo Fernández JA. Topical issues in venous thromboembolism. Drugs 2010; 70 Suppl. 2: 11–8
Glaxo Group Ltd. Arixtra solution for injection, pre-filled syringe: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000403/WC500027746.pdf [Accessed 2012 May 29]
Sweetman SC. Martindale thirty-sixth edition: the complete drug reference. London and Chicago: Pharmaceutical Press, 2009
Wilke T, Müller S. Nonadherence in outpatient thromboprophylaxis after major orthopedic surgery: a systematic review. Expert Rev Pharmacoecon Outcomes Res 2010 Dec; 10 (6): 691–700
Bayer Schering Pharma AG. Xarelto film-coated tablets: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf [Accessed 2012 May 29]
Boehringer Ingelheim International GmbH. Pradaxa hard capsules: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf [Accessed 2012 May 29]
Friedman RJ. Novel oral anticoagulants for VTE prevention in orthopedic surgery: overview of phase 3 trials. Orthopedics 2011 Oct; 34 (10): 795–804
Wittkowsky AK. Novel oral anticoagulants and their role in clinical practice. Pharmacotherapy 2011 Dec; 31 (12): 1175–91
Rupprecht HJ, Blank R. Clinical pharmacology of direct and indirect factor Xa inhibitors. Drugs 2010 Nov 12; 70 (16): 2153–70
Weitz JI. New oral anticoagulants in development. Thromb Haemost 2010 Jan; 103 (1): 62–70
Van Es J, Eerenberg ES, Kamphuisen PW, et al. How to prevent, treat, and overcome current clinical challenges of VTE. J Thromb Haemost 2011 Jul; 9 Suppl. 1: 265–74
Gross PL, Weitz JI. New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol 2008 Mar; 28 (3): 380–6
National Institute for Health and Clinical Excellence. Final appraisal determination: apixaban for the prevention of venous thromboembolism after total hip or knee replacement in adults [online]. Available from URL: http://www.nice.org.uk/nicemedia/live/12795/57280/57280.pdf [Accessed 2012 Jan 18]
Janssen Pharmaceuticals, Inc. Xarelto (rivaroxaban) tablets: US prescribing information [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202439s001lbl.pdf [Accessed 2012 May 29]
Duggan ST, Scott LJ, Plosker GL. Rivaroxaban: a review of its use for the prevention of venous thromboembolism after total hip or knee replacement surgery. Drugs 2009; 69 (13): 1829–51
Huo MH. New oral anticoagulants in venous thromboembolism prophylaxis in orthopaedic patients: are they really better?. Thromb Haemost 2011 Jul; 106 (1): 45–57
Watson J, Whiteside G, Perry C. Apixaban: first global approval. Drugs 2011 Oct 22; 71 (15): 2079–89
Maratea D, Fadda V, Trippoli S, et al. Prevention of venous thromboembolism after major orthopedic surgery: indirect comparison of three new oral anticoagulants. J Thromb Haemost 2011 Sep; 9 (9): 1868–70
Mantha S. Oral factor Xa inhibitors vs. enoxaparin for thromboprophylaxis after joint replacement surgery: a meta-analysis [abstract no. P-MO-419]. J Thromb Haemost 2011; 9 Suppl. 2: 189
Gómez-Outes A, Terleira-Fernández A, Suárez-Gea ML, et al. New oral anticoagulants for thromboprophylaxis after total hip or knee replacement: a meta-analysis and indirect treatment comparisons [abstract no. 08]. Basic Clin Pharmacol Toxicol 2011; 109 Suppl. 3: 34
Ruppert A, Steinle T, Lees M. Economic burden of venous thromboembolism: a systematic review. J Med Econ 2011; 14 (1): 65–74
Migliaccio-Walle K, Rublee D, Simon TA. Anticoagulation prophylaxis in orthopedic surgery: an efficacy frontier approach. Postgrad Med 2012 Jan; 124 (1): 41–9
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: T.L. Carman, Harrington Heart and Vascular Institute, University Hospitals Case Medical Center, Cleveland, OH, USA; W.D. Fisher, Department of Surgery, McGill University Health Centre, Montreal, QC, Canada; J. Harenberg, Department of Clinical Pharmacology, Medical Faculty, Ruprecht-Karls-University Heidelberg, Mannheim, Germany; M.R. Lassen, Center of Excellence for Spine Disease, Glostrup Hospital, University of Copenhagen, Glostrup, Denmark; J.A. Paramo Fernandez, Laboratory of Atherosclerosis, Area of Cardiovascular Sciences, Hematology and Hemotherapy Service, Center for Applied Medical Research, Pamplona Navarra University Clinic, Pamplona, Spain; A.G.G. Turpie, Hamilton Health Sciences, McMaster University, Hamilton, ON, Canada.
Data Selection
Sources:Medical literature (including published and unpublished data) on ‘apixaban’ was identified by searching databases (including MEDLINE and EMBASE) for articles published since 1996, bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the company developing the drug.
Search strategy: MEDLINE and EMBASE search terms were ‘apixaban’ and (‘venous thromboembolism’ or ‘thromboembolism’ or ‘venous thrombosis’ or ‘deep vein thrombosis’ or ‘thromboprophylaxis and surgery’ or ‘arthroplasty, replacement, hip’ or ‘hip replacement’ or ‘hip arthroplasty’ or ‘arthroplasty, replacement, knee’ or ‘knee replacement’ or ‘knee arthroplasty’ or ‘arthroplasty’ or ‘orthopaedic surgery’ or ‘orthopedic surgery’ or ‘orthopaedics’ or ‘orthopedics’). Searches were last updated 14 May 2012.
Selection: Studies in patients undergoing major orthopaedic surgery who received apixaban. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Apixaban, venous thromboembolism, deep vein thrombosis, pulmonary embolism, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Deeks, E.D. Apixaban. Drugs 72, 1271–1291 (2012). https://doi.org/10.2165/11209020-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11209020-000000000-00000