Abstract
Subcutaneous recombinant interferon-β-1a (SC IFNβ-1a) [Rebif®] is indicated as monotherapy for the prevention of relapses and progression of physical disability in patients with relapsing multiple sclerosis (MS). This article reviews the efficacy and tolerability of SC IFNβ-1a in this indication, with further discussion of its pharmacological properties and pertinent pharmacoeconomic studies.
SC IFNβ-1a efficacy and tolerability were evaluated in randomized, double-blind, multinational trials in patients with relapsing-remitting MS (RRMS). Its efficacy was demonstrated in the 2-year PRISMS trial, as SC IFNβ-1a 22 or 44 µg three times weekly (tiw) significantly reduced relapse rates, with an ≈30% relative risk reduction compared with placebo. SC IFNβ-1a was also associated with significantly delayed progression of disability, and lower disease activity according to MRI, relative to placebo. In the 24-week EVIDENCE trial, a significantly higher proportion of SC IFNβ-1a 44 µg tiw than intramuscular IFNβ-1a (Avonex®) 30 µg once weekly recipients remained relapse free. A serum-free formulation of SC IFNβ-1a 44µg tiw was more efficacious than placebo in preventing the development of brain lesions in the 16-week IMPROVE trial. In the 96-week REGARD trial, the efficacy of SC IFNβ-1a 44 µg tiw was not significantly different to that of glatiramer acetate for clinical endpoints, although it was associated with reduced development of brain lesions compared with glatiramer acetate, according to some MRI end-points.
In the 36-month CAMMS223 trial, alemtuzumab led to significantly lower relapse rates and risk of developing sustained disability than SC IFNβ-1a 44 µg tiw, and was generally more efficacious according to other clinical and MRI endpoints.
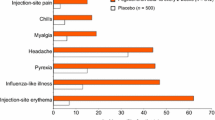
Across trials, influenza-like symptoms, injection-site reactions, haematological disturbances and hepatic enzyme abnormalities were the most common treatment-emergent adverse events occurring with SC IFNβ-1a. In the PRISMS trial, SC IFNβ-1a 22 and 44µg tiw recipients had more injection-site reactions than placebo recipients and, at the higher dosage, haematological disturbances and increases in ALT levels were also significantly more frequent than with placebo. Pooled data from clinical trials and postmarketing surveillance indicate that haematological and hepatic adverse events are generally asymptomatic and rarely result in treatment discontinuation. Nevertheless, some cases of serious hepatic complications have been reported.
In cost-utility studies, first-line therapies for RRMS, including SC IFNβ-1a, all exceeded commonly accepted US thresholds for incremental cost per quality-adjusted life-years gained relative to symptomatic treatment. However, because of patient need and the difficulty in adequately assessing cost utility in a gradually progressive disease, these agents have been made available to many patients worldwide through special access programmes.
Overall, SC IFNβ-1a has a favourable risk-benefit ratio and is a valuable first-line treatment option for patients with relapsing MS.









Similar content being viewed by others
References
Calabrese M, Filippi M, Gallo P. Cortical lesions in multiple sclerosis. Nature Rev Neurosci 2010; 6(8): 438–44
World Health Organization. Atlas: multiple sclerosis resources in the world [online]. Available from URL: http://www.who.int/mental_health/neurology/Atlas_MS_WEB.pdf [Accessed 2011 May 12]
Tremlett H, Zhao Y, Rieckmann P, et al. New perspectives in the natural history of multiple sclerosis. Neurology 2010 Jun 15; 74(24): 2004–15
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald Criteria. Ann Neurol 2011 Feb; 69(2): 292–302
Ramagopalan SV, Dobson R, Meier UC, et al. Multiple sclerosis: risk factors, prodromes and potential causal pathways. Lancet Neurol 2010 Jul; 9(7): 727–39
Goodin DS. Disease-modifying therapy in multiple sclerosis: update and clinical implications. Neurology 2008 Dec 9; 71 (24 Suppl. 3): S8–13
European Medicines Agency. Rebif 44 micrograms solution for injection in pre-filled syringe: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000136/WC500048681.pdf [Accessed 2011 May 18]
EMD Serono. Rebif® (interferon beta 1a sc injection): US prescribing information [online]. Available from URL: http://www.emdserono.com/cmg.emdserono_us/en/images/rebif_tcm115_19765.pdf. [Accessed 2011 May 10]
Merck Serono. Online report 2010 [online]. Available from URL: http://merck.online-report.eu/2010/ar/servicepages/downloads/files/merck_serono_merck_ar10.pdf [Accessed 2011 May 17]
McKeage K, Wagstaff AJ. Subcutaneous interferon-beta-1a: new formulation. CNS Drugs 2007; 21(10): 871–6
Giovannoni G, Barbarash O, Casset-Semanaz F, et al. Safety and immunogenicity of a new formulation of interferon beta-1a (Rebif New Formulation) in a Phase IIIb study in patients with relapsing multiple sclerosis: 96-week results. Mult Scler 2009 Feb; 15(2): 219–28
The Merck Group. New formulation of multiple sclerosis treatment Rebif® approved in European Union [media release]. 2007 Aug 30 [online]. Available from URL: http://news.merck.de/N/0/6BD040FBB8A250EFC12573460047FF38/$File/Rebif-New.pdf [Accessed 2011 May 10]
The Merck Group. Merck KGaA launches RebiSmart™, first electronic injection device for delivery of multiple sclerosis treatment Rebif® [media release]. 2009 Jun 24 [online]. Available from URL: http://www.merck.de/en/media/extNewsDetail.html?newsId=FB414ED6A2523C91C12575DE004DAFE0&newsType=1 [Accessed 2011 May 10]
Wagstaff AJ, Goa KL. Recombinant interferon-beta-1a: a review of its therapeutic efficacy in relapsing-remitting multiple sclerosis. Biodrugs 1998; 10(6): 471–94
Murdoch D, Lyseng-Williamson KA. Subcutaneous recombinant interferon-beta-1a (Rebif®): a review of its use in relapsing-remitting multiple sclerosis. Drugs 2005; 65(9): 1295–312
Chofflon M. Mechanisms of action for treatments in multiple sclerosis: does a heterogeneous disease demand a multitargeted therapeutic approach? Biodrugs 2005; 19(5): 299–308
Markowitz CE. Interferon-beta: mechanism of action and dosing issues. Neurology 2007 Jun 12; 68 Suppl. 4: S8–11
Antonetti F, Finocchiaro O, Mascia M, et al. A comparison of the biologic activity of two recombinant IFN-beta preparations used in the treatment of relapsing-remitting multiple sclerosis. J Interferon Cytokine Res 2002 Dec; 22(12): 1181–4
Kauffman MA, Yankilevich P, Barrero P, et al. Whole genome analysis of the action of interferon-beta. Int J Clin Pharmacol Ther 2009 May; 47(5): 328–57
Noronha A, Toscas A, Jensen MA. Interferon beta decreases T cell activation and interferon gamma production in multiple sclerosis. J Neuroimmunol 1993 Jul; 46(1–2): 145–53
Zhang X, Jin J, Tang Y, et al. IFN-beta1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J Immunol 2009 Mar 15; 182(6): 3928–36
Graber J, Zhan M, Ford D, et al. Interferon-beta-1a induces increases in vascular cell adhesion molecule: implication for its mode of action. J Neuroimmunol 2005 Apr; 161(1–2): 169–76
Korporal M, Haas J, Balint B, et al. Interferon beta-induced restoration of regulatory T-cell function in multiple sclerosis is prompted by an increase in newly generated naive regulatory T cells. Arch Neurol 2008 Nov 1; 65(11): 1434–9
de Andres C, Aristimuno C, Bartolome M, et al. Clinical response to interferon-beta-1a may be linked to low baseline circulating BDCA1 myeloid dendritic cells. Differential role of circulating dendritic cells and CD4+ regulatory T-cells in relapsing-remitting multiple sclerosis: a 1-year longitudinal study. J Neuroimmunol 2009 Jul 25; 212(1–2): 112–20
de Andres C, Aristimuno C, de las Heras V, et al. Interferon beta-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosis. J Neuroimmunol 2007 Jan; 182(1–2): 204–11
Bertolotto A, Gilli F, Sala A, et al. Persistent neutralizing antibodies abolish the interferon X bioavailability in MS patients. Neurology 2004 Jun; 60(4): 634–9
Sorenson PS, Ross C, Clemmesen KM. Clinical importance of neutralizing antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. Lancet 2003 Oct 11; 362(9391): 1184–91
Francis GS, Rice GP, Alsop JC. Interferon beta-1a in MS: results following development of neutralizing antibodies in PRISMS. Neurology 2005; 65(1): 48–55
Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon β-1a treatment regimens in MS: the EVIDENCE trial. Neurology 2002; 59(10): 1496–506
Buchwalder PA, Buclin T, Trinchard I, et al. Pharmacokinetics and pharmacodynamics of IFNβ-1a in healthy volunteers. J Interferon Cytokine Res 2000 Oct; 20(10): 857–66
Brearley C, Jaber A, Bertolino M, et al. Assessment of the safety, tolerability, and PK/PD properties of two new formulations of subcutaneously administered IFN-β1a: a double-blind, placebo-controlled comparison with the currently available formulation. Int J Clin Pharmacol Ther 2007; 45(6): 307–18
PRISMS (Prevention of Relapses and disability by Interferon β-1a Subcutaneously in Multiple Sclerosis). Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352(7): 1498–504
Sorensen PS, Mellgren SI, Svenningsson A, et al. Nordic trial of oral methylprednisolone as add-on therapy to interferon beta-1a for treatment of relapsing-remitting multiple sclerosis (NORMIMS study): a randomised, placebo-controlled trial. Lancet Neurol 2009 Jun; 8(6): 519–29
De Stefano N, Curtin F, Stubinski B, et al. Rapid benefits of a new formulation of subcutaneous interferon beta-1a in relapsing-remitting multiple sclerosis. Mult Scler 2010 Jul; 16(7): 888–92
Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the Rebif vs glatiramer acetate in relapsing MS disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol 2008 Oct; 7(10): 903–14
CAMMS223 Trial Investigators. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008 Oct 23; 359(17): 1786–801
The PRISMS (Prevention of Relapses and disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) study group. PRISMS-4: long-term efficacy of interferon-β-1a in relapsing MS. Neurology 2001 Jun; 56(12): 1628–36
Schwid SR, Panitch HS. Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clin Ther 2007 Sep; 29(9): 2031–48
SPECTRIMS Study Group. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: clinical results. Neurology 2001 Jun; 56(11): 1496–504
Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983 Nov; 33(11): 1444–52
Li DK, Paty DW. Magnetic resonance imaging results from the PRISMS trial: a randomized, double-blind, placebo-controlled study of interferon-β-1a in relapsing-remitting multiple sclerosis. Ann Neurol 1999 Aug; 46(2): 197–206
Cohen BA, Rivera VM. PRISMS: the story of a pivotal clinical trial series in multiple sclerosis. Curr Med Res Opin 2010 Apr; 26(4): 827–38
Coyle P, Singer B, Cohen B, et al. Efficacy of subcutaneous interferon beta-1a in patients with early-stage multiple sclerosis enrolled in the Prevention of Relapses and disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis study: results of a post hoc analysis [abstract no. p780]. 25th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; 2009 Sep 9–12; Düsseldorf
Oger J, Francis G, Chang P, et al. Prospective assessment of changing from placebo to IFN beta-1a in relapsing MS: the PRISMS study. J Neurol Sci 2005 Oct 15; 237(1–2): 45–52
Kappos L, Traboulsee A, Constantinescu C, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology 2006; 67(6): 944–53
Li DKB, Zhao GJ, Paty DW. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: MRI results. University of British Columbia MS/MRI Analysis Research Group and the SPECTRIMS Study Group. Neurology 2001 Jun 12; 56(11): 1505–13
Schwid SR, Thorpe J, Sharief M, et al. Enhanced benefit of increasing interferon beta-1a dose and frequency in relapsing MS: the EVIDENCE study. Arch Neurol 2005 May; 62(5): 785–92
Camu W, Hadjout K, Latour S, et al. Patient satisfaction following transition from the original to the new formulation of subcutaneous interferon beta-1a in relapsing multiple sclerosis: a randomized, two-arm, open-label, phase IIIb study. Patient Prefer Adherence 2010; 4: 127–33
Francis GS, Grumser Y, Alteri E, et al. Hepatic reactions during treatment of multiple sclerosis with interferon-β-1a: incidence and clinical significance. Drug Saf 2003; 26(11): 815–27
Rieckmann P, O’Connor P, Francis GS, et al. Haematological effects of interferon-β-1a (Rebif®) therapy in multiple sclerosis. Drug Saf 2004; 27(10): 745–56
Sandberg-Wollheim M, Alteri E, Moraga MS, et al. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler 2011 Apr; 17(4): 423–30
Sandberg-Wollheim M, Kormann G, Bischof D, et al. The risk of malignancy is not increased in patients with multiple sclerosis treated with subcutaneous interferon beta-1a: analysis of data from clinical trial and post-marketing surveillance settings. Mult Scler 2011 Apr; 17(4): 431–40
Guo S, Bozkaya D, Ward A, et al. Treating relapsing multiple sclerosis with subcutaneous versus intramuscular interferon-beta-1a: modelling the clinical and economic implications. Pharmacoeconomics 2009; 27(1): 39–53
Bell C, Graham J, Earnshaw S, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm 2007 Apr; 13(3): 245–61
Tappenden P, McCabe C, Chilcott J, et al. Cost-effectiveness of disease-modifying therapies in the management of multiple sclerosis for the Medicare population. Value Health 2009; 12(5): 657–65
Goldberg LD, Edwards NC, Fincher C, et al. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manage Care Pharm 2009 Sep; 15(7): 543–55
Nuijten M, Mittendorf T. A health-economic evaluation of disease-modifying drugs for the treatment of relapsing-remitting multiple sclerosis from the German societal perspective. Clin Ther 2010 Apr; 32(4): 717–28
Noyes K, Bajorska A, Chappel A, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: a population-based study. Neurology 2011; 77(4): 355–63
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005 Aug 4; 353(5): 487–97
Reynolds MW, Stephen R, Seaman C, et al. Persistence and adherence to disease modifying drugs among patients with multiple sclerosis. Curr Med Res Opin 2010 Mar; 26(3): 663–74
Wray S, Armstrong R, Cascione M, et al. A single-use autoinjector for self-administration of subcutaneous interferon beta-1a: final results from MOSAIC [abstract no. P909]. 26th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; 2010 Oct 13–16; Göteborg
Lugaresi A, Durastanti V, Gasperini C, et al. Safety and tolerability in relapsing-remitting multiple sclerosis patients treated with high-dose subcutaneous interferon-beta by Rebiject autoinjection over a 1-year period: the CoSa study. Clin Neuropharmacol 2008 May–Jun; 31(3): 167–72
Devonshire V, Arbizu T, Borre B, et al. Patient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: an international, single-arm, multicentre, phase IIIb study. BMC Neurol 2010 Apr 30; 10(1): 28
Rudick RA, Cohen JA, Weinstock-Guttman B, et al. Management of multiple sclerosis. N Engl J Med 1997 Nov 27; 337(22): 1604–11
Menge T, Weber MS, Hemmer B, et al. Disease-modifying agents for multiple sclerosis: recent advances and future prospects. Drugs 2008 Dec 3; 68(17): 2445–68
Gold R. Oral therapies for multiple sclerosis. CNS Drugs 2011 Jan 1; 25(1): 37–52
Carter NJ, Keating GM. Glatiramer acetate: a review of its use in relapsing-remitting multiple sclerosis and in delaying onset of clinically definite multiple sclerosis. Drugs 2010; 70(12): 1545–77
Bielekova B, Becker BL. Monoclonal antibodies in MS: mechanisms of action. Neurology 2010 Jan; 74 Suppl. 1: S31–40
Association of British Neurologists. Revised (2009) Association of British Neurologists guidelines for prescribing in multiple sclerosis [online]. Available from URL: http://www.theabn.org/abn/userfiles/file/ABN_MS_Guidelines_ 2009_Final(1).pdf [Accessed 2011 Jun 1]
Cossburn M, Pace AA, Jones J, et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology 2011 Aug 9; 77(6): 573–9
Uitdehaag BM, Barkhof F, Coyle PK, et al. The changing face of multiple sclerosis clinical trial populations. Curr Med Res Opin 2011 Aug; 27(8): 1529–37
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010 Feb 4; 362(5): 402–15
Galetta SL, Markowitz C. US FDA-approved disease-modifying treatments for multiple sclerosis: review of adverse effect profiles. CNS Drugs 2005; 19(3): 239–52
Ravnborg M, Sorensen PS, Andersson M, et al. Methyl-prednisolone in combination with interferon beta-1a for relapsing-remitting multiple sclerosis (MECOMBIN study): a multicentre, double-blind, randomised, placebo-controlled, parallel-group trial. Lancet Neurol 2010 Jul; 9(7): 672–80
Stuve O, Bennett JL, Hemmer B, et al. Pharmacological treatment of early multiple sclerosis. Drugs 2008; 68(1): 73–83
Comi G, De Stefano N, Freedman MS, et al. Efficacy of two dosing frequencies of subcutaneous interferon beta-1a on risk to conversion to multiple sclerosis in patients with clinically isolated syndrome: results of a phase III, randomized, double-blind, placebo-controlled, multicentre trial (REFLEX) [abstract no. P07.194 plus poster]. 63rd Annual Meeting of the American Academy of Neurology; 2011 Apr 9–16; Honolulu (HA)
Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon-beta-1b delays conversion to clinically-definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006; 67(7): 1242–9
Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 2000; 343(13): 898–904
Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 2001; 357(9268): 1576–82
Comi G, Martinelli V, Rodegher M, et al. Effect of glatirmaer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 2009; 374(9700): 1503–11
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanford, M., Lyseng-Williamson, K.A. Subcutaneous Recombinant Interferon-β-1a (Rebif®). Drugs 71, 1865–1891 (2011). https://doi.org/10.2165/11207540-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11207540-000000000-00000