Abstract
The corticosteroid mometasone and the long-acting β2-selective adrenoreceptor agonist formoterol have been combined in a single pressurized metered-dose inhaler for use in patients aged ≥12 years with asthma.
In a 26-week well designed trial in patients with persistent asthma uncontrolled on medium-dose inhaled corticosteroids (ICS), mometasone/formoterol 200 μg/10 μg twice daily (bid) was more effective than placebo or the same nominal dosage of formoterol alone in reducing the incidence of asthma deteriorations, as well as in improving lung function, asthma control, asthma symptoms and asthma-related quality-of-life outcomes. The combination was also more effective than the same nominal dosage of mometasone alone in improving lung function and asthma control.
Similarly, in a 12-week well designed trial in patients with persistent asthma uncontrolled on high-dose ICS, mometasone/formoterol 400μg/10μg bid was more effective than the same nominal dosage of mometasone alone in improving lung function, asthma control and asthma symptoms. Treatment with a lower dosage of the combination (200μg/10μg bid) yielded similar results and, moreover, significantly reduced the incidence of asthma deteriorations compared with mometasone alone.
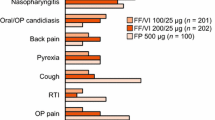
Mometasone/formoterol was generally well tolerated in clinical trials of 12–52 weeks’ duration. The adverse event profile of the combination was consistent with that of its individual components; no new or unexpected safety signals were detected.





Similar content being viewed by others
References
Fanta CH. Asthma. N Engl J Med 2009; 360 (10): 1002–9
World Health Organisation. Asthma fact sheet no. 307 [online]. Available from URL: http://www.who.int/mediacentre/factsheets/fs307/en/index.html [Accessed 2011 Sep 27]
Global Initiative for Asthma. Global burden of asthma™ [online]. Available from URL: http://www.ginasthma.org/uploads/users/files/backgrounder-20.pdf [Accessed 2010 Nov 22]
National Heart LaBI. US Department of Health and Human Services. National Asthma Education and Prevention Program. Expert Panel report 3: guidelines for the diagnosis and management of asthma. Summary report 2007 [online]. Available from URL: http://www.nhlbi.nih.gov/guidelines/asthma/asthsumm.pdf [Accessed 2010 Nov 22]
Blair R, Breit JS, Peters SP. The Merck Manuals Online Medical Library: asthma [online]. Available from URL: http://www.merckmanuals.com/home/sec04/ch044/ch044a.html [Accessed 2011 Sep 27]
Papiris SA, Manali ED, Kolilekas L, et al. Acute severe asthma: new approaches to assessment and treatment. Drugs 2009; 69 (17): 2363–91
Fuhlbrigge AL, Adams RJ, Guilbert W, et al. The burden of asthma in the United States. Am J Resp Crit Care Med 2002; 166: 1044–9
Chung KF, Caramori G, Adcock IM. Inhaled corticosteroids as combination therapy with beta-adrenergic agonists in airways disease: present and future. Eur J Clin Pharmacol 2009 Sep; 65 (9): 853–71
Postma DS, Kerstjens HA, ten Hacken NH. Inhaled corticosteroids and long-acting beta-agonists in adult asthma: a winning combination in all?. Naunyn Schmiedeberg Arch Pharmacol 2008; 378 (2): 203–15
Frois C, Wu EQ, Ray S, et al. Inhaled corticosteroids or long-acting beta-agonists alone or in fixed-dose combinations in asthma treatment: a systematic review of fluticasone/budesonide and formoterol/salmeterol. Clin Ther 2009 Dec; 31 (12): 2779–803
Sharpe M, Jarvis B. Inhaled mometasone furoate: a review of its use in adults and adolescents with persistent asthma. Drugs 2001; 61 (9): 1325–50
Faulds D, Hollingshead LM, Goa KL. Formoterol: a review of its pharmacological properties and therapeutic potential in reversible obstructive airway disease. Drugs 1991; 42 (1): 115–37
Goldsmith DR, Keating GM. Budesonide/formoterol: a review of its use in asthma. Drugs 2004; 64 (14): 1597–618
Affrime MB, Cuss F, Padhi D, et al. Bioavailability and metabolism of mometasone furoate following administration by metered-dose and dry-powder inhalers in healthy human volunteers. J Clin Pharmacol 2000; 40: 1227–36
Affrime MB, Kosoglou T, Thonoor CM, et al. Mometasone furoate has minimal effects on the hypothalamic-pituitary-adrenal axis when delivered at high doses. Chest 2000; 118: 1538–46
Lecaillon JB, Kaiser G, Palmisano M, et al. Pharmacokinetics and tolerability of formoterol in healthy volunteers after a single high dose of Foradil dry powder inhalation via aerolizer™. Eur J Clin Pharmacol 1999; 55: 131–8
Fan Y, Zhao L, Roy P. NDA22-518: clinical pharmacology review [online]. Available from URL: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM224594.pdf [Accessed 2011 Sep 27]
Asmanex Twisthaler 110mcg,220mcg(mometasonefuroate inhalation powder): US prescribing information. Kenilworth (NJ): Schering Corporation, a subsidiary of Schering-Plough Corporation, 2010
Foradil® Aerolizer® (formoterol fumarate inhalation powder): US prescribing information. Kenilworth (NJ): Schering Corporation, 2002
Dulera® 100mcg/5mcg (mometasone furoate 100mcg and formoterol fumarate dihydrate 5 mcg) inhalation aerosol; Dulera® 200 mcg/5 mcg (mometasone furoate 200 mcg and formoterol fumarate dihydrate 5 mcg) inhalation aerosol: US prescribing information. Whitehouse (NJ): Schering Corporation, a subsidiary of Merck & Co., Inc., 2010
Maspero JF, Nolte H, Cherrez-Ojeda I, et al. Long-term safety of mometasone furoate/formoterol combination for treatment of patients with persistent asthma. J Asthma 2010; 47: 1106–15
Weinstein SF, Corren J, Murphy K, et al. Twelve-week efficacy and safety study of mometasone furoate/formoterol 200/10μg and 400/10μg combination treatments in patients with persistent asthma previously receiving high-dose inhaled corticosteroids. Allergy Asthma Proc 2010; 31 (4): 280–9
Nathan RA, Nolte H, Pearlman DS. Twenty-six-week efficacy and safety study of mometasone furoate/formoterol 200/10 μg combination treatment in patients with persistent asthma previously receiving medium-dose inhaled corticosteroids. Allergy Asthma Proc 2010; 31 (4): 269–79
Meltzer EO, Kuna P, Nolte H, et al. Mometasone furoate/formoterol reduces asthma deteriorations and improves lung function. Eur Respir J. Epub 2011 Aug 4
Weinstein SF, Nathan RA, Nolte H. Combined mometasone furoate/formoterol reduces asthma deteriorations in patients with persistent asthma uncontrolled on medium-or high-dose inhaled corticosteroids [abstract no. P89]. Ann Allergy Asthma Immunol 2010 Nov; 105 (5 Suppl. 1): A51-2
White M, Meltzer EO, Nathan RA, et al. Improved asthma control with mometasone furoate/formoterol: a new combination treatment for persistent asthma [abstract no. P83]. Ann Allergy Asthma Immunol 2010 Nov; 105 (5 Suppl. 1): A49-50
Murphy K, Meltzer EO, Nathan RA, et al. Quality of life improvements in persistent asthma subjects receiving combined mometasone furoate and formoterol [abstract no. P88]. Ann Allergy Asthma Immunol 2010 Nov; 105 (5 Suppl. 1): A51
Nolte H, Maspero J, Cherrez Ojeda I. The safety and tolerability characteristics of mometasone furoate/formoterol (MF/F) combination therapy in patients with persistent asthma: findings from the MF/F phase III clinical development program [abstract no. 275 plus poster]. 75th Annual International Scientific Assembly of the American College of Chest Physicians; 2009 31 Oct–5 Nov; San Diego (CA)
Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteriods for treating asthma. N Engl J Med 2011 Jun 30; 364 (26): 2473–5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frampton, J.E. Mometasone/Formoterol Inhalation Aerosol. Drugs 72, 1229–1241 (2012). https://doi.org/10.2165/11206920-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11206920-000000000-00000