Abstract
The pneumococcal polysaccharide conjugate vaccine Prevenar 13® (PCV13) comprises 13 capsular Streptococcus pneumoniae polysaccharide serotypes that are individually conjugated to nontoxic diphtheria protein (cross-reactive material [CRM197]).
In randomized, comparator-controlled, phase III trials in healthy infants aged 2–6 months, PCV13 elicited a strong immune response against all 13 pneumococcal serotypes in terms of the proportion of vaccinees achieving reference antibody levels with a two- or three-dose primary vaccination series. Immune responses for the seven serotypes common to PCV13 and the 7-valent pneumococcal conjugate vaccine Prevenar® (PCV7) were generally similar. Antibodies to all vaccine serotypes were functional.
A booster dose of PCV13 administered between 11 and 15 months of age generally boosted the immune response against all 13 serotypes, regardless of whether infants had previously received PCV13 or PCV7 during the primary vaccination phase. Robust immune responses against all serotypes were achieved when PCV13 was administered as catch-up vaccination schedules in older infants and young children aged 7–72 months.
Importantly, PCV13 did not interfere with the immune responses to coadministered routine paediatric vaccines.
Based on data for PCV7, it is expected that PCV13 will also display protective efficacy against invasive pneumococcal disease, otitis media and pneumonia.
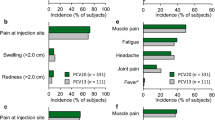
PCV13 was generally well tolerated, with an adverse event profile similar to that of PCV7 after any vaccine dose.
Similar content being viewed by others
References
O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009 Sep 12; 374(9693): 893–902
World Health Organization. Acute respiratory infections (update September 2009) [online]. Available from URL: http://www.who.int/vaccine_research/diseases/ari/en/print.html [Accessed 2010 May 13]
European Medicines Agency. Assessment report for Prevenar 13 [online]. Available from URL: http://www.ema.europa.eu/humandocs/PDFs/EPAR/Prevenar13/H-1104-en6.pdf [Accessed 2010 Apr 6]
Hausdorff WP, Bryant J, Paradiso PR, et al. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis 2000 Jan; 30(1): 100–21
Henrichsen J. Typing of Streptococcus pneumoniae: past, present, and future. Am J Med 1999 Jul 26; 107(1A): 50S–4S
Ardanuy C, Fenoll A, Rolo D, et al. Characterization of serotype 5 pneumococci causing an outbreak of invasive pneumococcal disease (IPD) in 2005 in Barcelona, Spain. 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2008 Oct 25–28; Washington, DC
Cohen R, Levy C, de La Rocque F, et al. Impact of pneumococcal conjugate vaccine and of reduction of antibiotic use on nasopharyngeal carriage of nonsusceptible pneumococci in children with acute otitis media. Pediatr Infect Dis J 2006 Nov; 25 (No. 11): 1001–7
Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003 May 1; 348(18): 1737–46
Millar EV, Watt JP, Bronsdon MA, et al. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin Infect Dis 2008 Oct 15; 47(8): 989–96
Isaacman DJ, Fletcher MA, Fritzell B, et al. Indirect effects associated with widespread vaccination of infants with heptavalent pneumococcal conjugate vaccine (PCV7; Prevnar). Vaccine 2007 Mar 22; 25(13): 2420–7
Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis 2007 Nov 1; 196(9): 1346–54
Hsu HE, Shutt KA, Moore MR, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med 2009 Jan 15; 360(3): 244–56
Nuorti P, Rosen J, Thomas A. Invasive pneumococcal disease in young children caused by serotypes in the new 13-valent conjugate vaccine: implications for immunization strategies [abstract no. G1-1554]. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009 Sep 12–15; San Francisco (CA)
Black S. The volatile nature of pneumococcal serotype epidemiology: potential for misinterpretation. Pediatr Infect Dis J 2010 Apr; 29(4): 301–3
O'Brien KL, Dagan R. The potential indirect effect of conjugate pneumococcal vaccines. Vaccine 2003 May 16; 21(17–18): 1815–25
Wyeth Pharmaceuticals Inc. Prevenar 13 suspension for injection; pneumococcal polyscaccharide conjugate (13-valent, adsorbed): summary of product characteristics [online]. Available from URL: http://www.medicines.org.uk/emc/document.aspx?documentid=22689&doctype=SPC [Accessed 2010 Apr 6]
Wyeth Pharmaceuticals Inc. Prevenar 13 (pneumococcal 13-valent conjugate vaccine [diphtheria CRM197 protein]): suspension for intramuscular injection [online]. Available from URL: http://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm201669.pdf [Accessed 2010 Jun 12]
Kieninger DM, Kueper K, Steul K, et al. Safety, tolerability and immunologic noninferiority of 13-valent pneumococcal conjugate vaccine compared to 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine 2010; 28(25): 4192–203
Hughes JY, Snape CL, Klinger CL, et al. Immunogenicity of booster doses of 13-valent pneumococcal conjugate and HIB/MENC vaccines given at 12 months of age in the UK. 27th Annual Meeting of the European Society for Paediatric Infectious Diseases; 2009 Jun 9–13; Brussels
Klinger CL, Snape MD, John T, et al. Immunogenicity of DTaP-IPV-Hib and menC vaccines in the UK when administered with a 13-valent Pneumococcal conjugate vaccine [abstract no. GI-2118]. 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy and 46th Annual Meeting of the Infectious Diseases Society of America; a joint meeting; 2008 Oct 25–28; Washington, DC: 367
Grimprel E, Laudat F, Baker SA, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine given with routine pediatric vaccination to healthy children in France. 27th Annual Meeting of the European Society for Paediatric Infectious Diseases; 2009 Jun 9–13; Brussels
Grimprel E, Scott D, Laudat F, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine given with routine pediatric vacination to healthy infants in France [abstract no. GI-2119]. 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy and 46th Annual Meeting of the Infectious Diseases Society of America: a joint meeting; 2008 Oct 25–28; Washington, DC
Gadzinowski J, Daniels E, Scott DA, et al. Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine with/without polysorbate 80 in healthy infants in Poland. 27th Annual Meeting of the European Society for Paediatric Infectious Diseases; 2009 Jun 9–13; Brussels
Esposito S, Tansey S, Thompson A, et al. Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine compared to 7-valent pneumococcal conjugate vaccine given as a 3-dose series with routine vaccines in healthy infants and toddlers. Clin Vaccine Immunol 2010; 17(6): 1017–26
Diez-Domingo J, Gurtman A, Bernaola E, et al. Safety and immunogenicity of 13-valent pneumococcal conjugate vaccine in healthy infants and toddlers receiving routine vaccinations in Spain. 27th Annual Meeting of the European Society for Paediatric Infectious Diseases; 2009 Jun 9–13; Brussels
Gadzinowski J, Tansey S, Mellelieu T, et al. A phase 3 trial evaluating the safety, tolerability and immunogenicity of manufacturing scale 13-valent pneumococcal conjugate vaccine [abstract no. GI-2116]. 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy and 46th Annual Meeting of the Infectious Diseases Society of America: a joint meeting; 2008 Oct 25–28; Washington, DC
Martinon-Torres F, Gimenez-Sanchez F, Gurtman A, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given with meningococcal C tetanus toxoid conjugate and other routine pediatric vaccinations [poster]. 28th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); 2010 May 4–8; Nice
Silfverdal AV, Tansey SP, Skoglund G, et al. Phase 3, openlabel trial of 13-valent pneumococcal conjugate vaccine as a toddler dose in healthy children previously partially immunized with 7-valent pneumococcal conjugate vaccine [poster]. 28th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); 2010 May 4–8; Nice
Wysocki J, Daniels ED, Sarkozy DA, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine administered to older infants and children naive to previous vaccination. 27th Annual Meeting of the European Society for Paediatric Infectious Diseases; 2009 Jun 9–13; Brussels
US Food and Drug Administration. Clinical review of biologics license application for Prevnar 13 (pneumococcal 13-valent conjugate vaccine [diphtheria CRM 197 protein]) [online]. Available from URL: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM206341.pdf [Accessed 2010 Sep 27]
World Health Organization. Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines [online]. Available from URL: http://www.who.int/biologicals/areas/vaccines/pneumo/Pneumo_final_23APRIL_2010.pdf [Accessed 2010 Jun 15]
Pavia M, Bianco A, Nobile CG, et al. Efficacy of pneumococcal vaccination in children younger than 24 months: a meta-analysis. Pediatrics 2009 Jun; 123(6): e1103–10
Patel R, Stoykova B, Lloyd AC, et al. A comparison of the cost-effectiveness of the 13-valent (PCV13) and 10-valent pneumococcal conjugate vaccines in the UK [abstract no. PIN57]. 12th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research; 2009 Oct 24; Paris, A248
Stoykova B, Strutton DR, Earnshaw SR, et al. Cost-effectiveness of 13-valent pneumococcal conjugate vaccination relative to 7-valent pneumococcal conjugate vaccination in the United Kingdom (UK). 27th Annual Meeting of the European Society for Paediatric Infectious Diseases; 2009 Jun 9–13; Brussels
Papanicolaou S, Kontodimas S, Syriopoulou V, et al. Clinical and economic benefits of national immunization with the 13-valent compared to 7- and 10-Valent Pneumococcal Conjugate Vaccines in Greece [abstract no. PIN35]. 12th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research; 2009 Oct 24; Paris, A423
Klok R, Strutton DR, Postma M, et al. Public health and economic impact of 13-valent pneumococcal conjugate vaccination in the Netherlands. 27th Annual Meeting of the European Society for Paediatric Infectious Diseases; 2009 Jun 9–13; Brussels
Strutton DR, Kuchenbecker U, Earnshaw SR, et al. Impact of 13-valent pneumococcal conjugate vaccination on costs and outcomes in Germany and the United States. 27th Annual Meeting of the European Society for Paediatric Infectious Diseases; 2009 Jun 9–13; Brussels
Whillans F, Kwan H, Strutton DR, et al. Cost-effectiveness of 13-valent and 10-valent pneumococcal conjugate vaccination relative to 7-valent pneumococcal conjugate vaccination in Canada [abstract no. PIN43]. 12th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research; 2009 Oct 24; Paris, A425
Patel R, Kuchenbecker U, Bowrin K, et al. The cost-effectiveness of 13-valent pneumococcal conjugate vaccine (PCV13) compared to 7-valent pneumococcal conjugate vaccine (PCV7) for childhood vaccination in Gerrmany. 27th Annual Meeting of the European Society for Paediatric Infectious Diseases; 2009 Jun 9–13; Brussels
Gurtman A, Tansey SP, Thompson A, et al. Safety of 13-valent pneumococcal conjugate vaccine in infants and children: meta-analysis of 13 clinical trials in 9 countries. Poster presented at the 28th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); 2010 May 4–8; Nice
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duggan, S.T. Pneumococcal Polysaccharide Conjugate Vaccine (13-Valent, Adsorbed) [Prevenar 13®]. Drugs 70, 1973–1986 (2010). https://doi.org/10.2165/11205110-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11205110-000000000-00000