Abstract
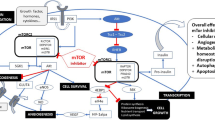
Temsirolimus selectively inhibits the mammalian target of rapamycin (mTOR) kinase, with subsequent inhibition of the translation of cell cycle regulatory proteins.
Therapy with intravenous temsirolimus 175 mg once weekly for 3 weeks followed by 75 mg once weekly (higher temsirolimus dosage), but not 25 mg once weekly (lower temsirolimus dosage), was significantly more effective than single-agent chemotherapy of the investigator’s choice in terms of the primary endpoint of progression-free survival (PFS), as assessed by independent review, in the treatment of adult patients with relapsed and/or refractory mantle cell lymphoma in a phase III study. Both dosage regimens of temsirolimus achieved significantly better outcomes with regard to PFS, as assessed by the investigator (secondary endpoint), than the investigator’s choice therapy.
Patients receiving the higher temsirolimus dosage achieved a significantly better outcome with regard to the objective response rate (ORR) than those receiving the investigator’s choice therapy; however, no significant difference in terms of ORR was observed between patients receiving the lower temsirolimus dosage and those receiving the investigator’s choice therapy. The differences between the two temsirolimus treatment groups and the investigator’s choice treatment group with regard to the endpoint of overall survival did not reach statistical significance.
The tolerability profile of temsirolimus in this patient population was mostly consistent with the known toxicities of the agent. The incidence of thrombocytopenia was significantly higher and that of leukopenia significantly lower in patients receiving the higher temsirolimus dosage compared with those receiving the investigator’s choice therapy. Adverse events were often managed with dose modifications.



Similar content being viewed by others
References
The Leukemia and Lymphoma Society. Mantle cell lymphoma [online]. Available from URL: http://www.leukemialymphoma.org/attachments/National/br_1172589724.pdf [Accessed 2010 Apr 15]
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-Hodgkin’s lymphomas. 1.2010 [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/nhl.pdf [Accessed 2010 Aug 23]
Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol 2009 Aug; 27(23): 3822–9
Gill S, Ritchie D. Therapeutic options in mantle cell lymphoma. Leuk Lymphoma 2008 Mar; 49(3): 398–409
Weigert O, Unterhalt M, Hiddemann W, et al. Current management of mantle cell lymphoma. Drugs 2007; 67(12): 1689–702
Coiffier B, Ribrag V. Exploring mammalian target of rapamycin (mTOR) inhibition for treatment of mantle cell lymphoma and other hematologic malignancies. Leuk Lymphoma 2009 Dec; 50(12): 1916–30
Hess G, Smith SM, Berkenblit A, et al. Temsirolimus in mantle cell lymphoma and other non-Hodgkin lymphoma subtypes. Semin Oncol 2009 Dec; 36 Suppl. 3: S37–45
Simpson D, Curran MP. Temsirolimus: in advanced renal cell carcinoma. Drugs 2008; 68(5): 631–8
European Medicines Agency. Torisel: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000799/WC500039912.pdf [Accessed 2010 Jul 9]
Hug B, Boni J, Leister C, et al. A single-dose, placebo- and moxifloxacin-controlled 3-period study of the effects of temsirolimus on cardiac repolarization in healthy subjects [abstract no. 2551]. J Clin Oncol 2009 May; 27 (15 Suppl.): 120s
Yazbeck VY, Buglio D, Georgakis GV, et al. Temsirolimus downregulates p21 without altering cyclin D1 expression and induces autophagy and synergizes with vorinostat in mantle cell lymphoma. Exp Hematol 2008; 36(4): 443–50
Singleton PA, Moss J, Garcia JG. Methylnaltrexone potentiates the antiangiogenic effects of the mTOR inhibitor, temsirolimus [abstract no. e14636]. J Clin Oncol 2009 May; 27(15Suppl.): e14636
European Medicines Agency. Torisel: assessment report [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000799/WC500039918.pdf [Accessed 2010 Apr 1]
Wyeth Pharmaceuticals Inc. Torisel® prescribing information [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022088s006lbl.pdf [Accessed 2010 May 5]
Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol 2005 Aug; 23(23): 5294–304
Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol 2005; 23(23): 5347–56
Ansell SM, Inwards DJ, Rowland Jr KM, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer 2008 Aug; 113(3): 508–14
Romaguera J, Coiffier B, Crump M, et al. Supportive efficacy analyses for the phase 3 study of temsirolimus versus investigator’s choice therapy for the treatment of patients with relapsed or refractory mantle cell lymphoma [abstract no. 1559]. Blood 2008 Dec; 112(11): 553. Plus poster presented at the 50th Annual Meeting and Exposition of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 2005 Aug; 23(23): 5314–22
Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007 May; 356(22): 2271–81
US National Institutes of Health. ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov/ct2/home [Accessed 2010 Jul 29]
Acknowledgements and Disclosures
This manuscript was reviewed by: G. Hess, Department of Haematology/Oncology, Johannes Gutenberg-University, Mainz, Germany; S.M. Smith, Section of Hematology/Oncology, The University of Chicago, Chicago, Illinois, USA.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoy, S.M., McKeage, K. Temsirolimus. Drugs 70, 1819–1829 (2010). https://doi.org/10.2165/11204940-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11204940-000000000-00000