Abstract
Tolvaptan is an orally administered, nonpeptide, selective arginine vasopressin V1 receptor antagonist that increases free water clearance, thereby correcting low serum sodium levels.
SALT-1 and -2, two identical, randomized, double-blind, placebo-controlled, multicentre trials, included patients with hypervolaemic or euvolaemic hyponatraemia (serum sodium <135 mmol/L) associated with heart failure, cirrhosis or the syndrome of inappropriate antidiuretic hormone secretion. In both trials, patients receiving (in addition to standard medical treatment) tolvaptan 15–60 mg once daily (titrated according to response) for up to 30 days (n=95 and 118) experienced significantly greater improvements than those receiving placebo (n = 89 and 114) for the co-primary endpoints of the change in average daily area under the curve for the serum sodium level from baseline to day 4 and from baseline to day 30.
This beneficial effect of tolvaptan on serum sodium levels in SALT-1 and -2 was observed in patients with mild (serum sodium <135 mmol/L) and in those with marked (serum sodium <130 mmol/L) hyponatraemia at baseline.
Tolvaptan was also superior to placebo in increasing serum sodium levels from baseline to day 7 in a subgroup of 323 patients with hyponatraemia (serum sodium <134 mmol/L) in the randomized, double-blind, multicentre EVEREST trials, which included patients who were hospitalized for worsening heart failure.
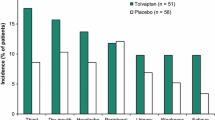
Tolvaptan was generally well tolerated in clinical trials. The most frequently reported adverse events were thirst and dry mouth, which result from the pharmacodynamic effects of the drug.


Similar content being viewed by others
References
Upadhyay A, Jaber BL, Madias NE. Epidemiology of hyponatremia. Semin Nephrol 2009 May; 29(3): 227–38
Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 2003 Nov 19; 290(19): 2581–7
Fraser JF, Stieg PE. Hyponatremia in the neurosurgical patient: epidemiology, pathophysiology, diagnosis, and management. Neurosurgery 2006 Aug; 59(2): 222–9
Schrier RW, Bansal S. Diagnosis and management of hyponatremia in acute illness. Curr Opin Crit Care 2008 Dec; 14(6): 627–34
Siragy HM. Hyponatremia, fluid-electrolyte disorders, and the syndrome of inappropriate antidiuretic hormone secretion: diagnosis and treatment options. Endocr Pract 2006; 12(4): 446–57
Ali F, Guglin M, Vaitkevicius P, et al. Therapeutic potential of vasopressin receptor antagonists. Drugs 2007; 67(6): 847–58
Cawley MJ. Hyponatremia: current treatment strategies and the role of vasopressin antagonists. Ann Pharmacother 2007 May; 41(5): 840–50
Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med 2000 May 25; 342(21): 1581–9
Verbalis JG, Goldsmith SR, Greenberg A, et al. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med 2007 Nov; 120 (11 Suppl. 1): S1–21
Samsca™ (tolvaptan): US prescribing information. Rockville (MD): Otsuka America Pharmaceutical Inc., 2009 May
Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 2008 May 10; 371(9624): 1624–32
Moen MD, Keating GM. Intravenous conivaptan. Am J Cardiovasc Drugs 2008; 8(5): 341–8
Costello-Boerrigter LC, Boerrigter G, Burnett Jr JC. Pharmacology of vasopressin antagonists. Heart Fail Rev 2009 Jun; 14(2): 75–82
Zhou K, Li H, Fong M, et al. Hypervolemic hyponatremia aggravates myocardial ischemia/reperfusion injury: normalization with tolvaptan [abstract no. 432]. Circulation 2006 Oct 31; 114 (18 Suppl. II): II–62
Shoaf SE, Wang Z, Bricmont P, et al. Pharmacokinetics, pharmacodynamics, and safety of tolvaptan, a nonpeptide AVP antagonist, during ascending single-dose studies in healthy subjects. J Clin Pharmacol 2007 Dec; 47(12): 1498–507
Costello-Boerrigter LC, Smith WB, Boerrigter G, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol 2006 Feb; 290(2): F273–8
Hauptman PJ, Zimmer C, Udelson J, et al. Comparison of two doses and dosing regimens of tolvaptan in congestive heart failure. J Cardiovasc Pharmacol 2005 Nov; 46(5): 609–14
Shoaf SE, Bramer SL, Bricmont P, et al. Pharmacokinetic and pharmacodynamic interaction between tolvaptan, a non-peptide AVP antagonist, and furosemide or hydrochlorothiazide. J Cardiovasc Pharmacol 2007 Aug; 50(2): 213–22
Shoaf SE, Ouyang J, Bricmont P, et al. Tolvaptan, a selective V2-receptor antagonist has no effect on QTCI following multiple oral dosing in healthy men and women [abstract no. PI-77]. Clin Pharmacol Ther 2009 Feb; 85 Suppl. 1: S32
Shoaf SE, Mallikaarjun S. Effect of ketoconazole and grapefruit juice, CYP3A4 inhibitors, on the pharmacokinetics of tolvaptan, a non-peptide vasopressin antagonist [abstract no. PII-49]. Clin Pharmacol Ther 2008 Mar; 83 Suppl. 1: S57
Samsca® (tolvaptan): EU summary of product characteristics. Uxbridge: Otsuka Pharmaceutical Europe Ltd, 2009
Shoaf SE, Mallikaarjun S. Pharmacokinetics and safety of tolvaptan, a new vasopressin antagonist, following single oral 60 to 480 mg doses in healthy subjects [abstract no. PI-79]. Clin Pharmacol Ther 2007 Mar 2; 81 Suppl. 1: 38
Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006 Nov 16; 355(20): 2099–112
Gheorghiade M, Konstam MA, Burnett Jr JC, et al. Shortterm clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007 Mar 28; 297(12): 1332–43
Konstam MA, Gheorghiade M, Burnett Jr JC, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007 Mar 28; 297(12): 1319–31
Pang PS, Konstam MA, Krasa HB, et al. Effects of tolvaptan on dyspnoea relief from the EVEREST trials. Eur Heart J 2009 Sep; 30(18): 2233–40
Blair JE, Khan S, Konstam MA, et al. Weight changes after hospitalization for worsening heart failure and subsequent re-hospitalization and mortality in the EVEREST trial. Eur Heart J 2009 Jul; 30(13): 1666–73
Blair JE, Zannad F, Konstam MA, et al. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol 2008 Nov 11; 52(20): 1640–8
Wang NC, Maggioni AP, Konstam MA, et al. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA 2008 Jun 11; 299(22): 2656–66
Gheorghiade M, Niazi I, Ouyang J, et al. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation 2003 Jun 3; 107(21): 2690–6
Udelson JE, Orlandi C, Ouyang J, et al. Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction: an international, multicenter, randomized, placebo-controlled trial. J Am Coll Cardiol 2008 Nov 4; 52(19): 1540–5
Udelson JE, McGrew FA, Flores E, et al. Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol 2007 Jun 5; 49(22): 2151–9
Gheorghiade M, Gattis WA, O’Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA 2004 Apr 28; 291(16): 1963–71
Gheorghiade M, Gottlieb SS, Udelson JE, et al. Vasopressin V2 receptor blockade with tolvaptan versus fluid restriction in the treatment of hyponatremia. Am J Cardiol 2006 Apr 1; 97(7): 1064–7
Acknowledgements and Disclosures
The manuscript was reviewed by: J.E.A. Blair, Northwestern University, Feinberg School of Medicine, Chicago, Illinois, USA; J.K. Ghali, Department of Cardiology, Detroit Receiving Hospital, Cardiovascular Clinical Trials Program DMC Cardiovascular Institute, Department of Medicine, Wayne State University, Detroit, Michigan, USA.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes based on any comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plosker, G.L. Tolvaptan. Drugs 70, 443–454 (2010). https://doi.org/10.2165/11204630-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11204630-000000000-00000