Abstract
A long-acting intramuscular formulation of the atypical antipsychotic agent risperidone is now indicated for the maintenance treatment of patients with bipolar I disorder.
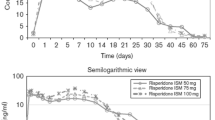
The formulation utilizes a novel drug delivery system of biodegradable microspheres and is bioequivalent to the oral formulation of the drug. Moreover, fluctuations in plasma drug concentrations at steady state were 1.7-fold lower with long-acting than with oral risperidone.
Maintenance treatment with risperidone long-acting injection, as monotherapy in adults with stabilized bipolar I disorder or as an adjunct to standard therapy in adults with stabilized, frequently relapsing bipolar I disorder, was effective in delaying relapse to symptoms in two well designed trials with maintenance phases of 1 or 2 years’ duration. The time to relapse to any mood episode (primary endpoint) was significantly longer with risperidone long-acting injection than with placebo in both studies.
Risperidone long-acting injection also significantly reduced the risk of relapse relative to placebo in these trials.
Maintenance treatment with risperidone long-acting injection was generally well tolerated in patients with bipolar disorder, both as monotherapy and adjunctive therapy, with most adverse reactions being of mild to moderate severity.




Similar content being viewed by others
References
Post RM. The impact of bipolar depression. J Clin Psychiatry 2005; 66 Suppl. 5: 5–10
Malhi GS, Adams D, Cahill CM, et al. The management of individuals with bipolar disorder: a review of the evidence and its integration into clinical practice. Drugs 2009; 69(15): 2063–101
World Health Organization. The world health report 2001 [online]. Available from URL: http://www.who.int/whr/2001/en/whr01_en.pdf [Accessed 2010 Feb 24]
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association, 1994
Yatham LN, Kennedy SH, Schaffer A, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2009. Bipolar Disord 2009 May; 11(3): 225–55
National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children and adolescents, in primary and secondary care [online]. Available from URL: http://www.nice.org.uk/nicemedia/pdf/CG38fullguideline.pdf [Accessed 2010 Feb 24]
Kemp DE, Canan F, Goldstein BI, et al. Long-acting risperidone: a review of its role in the treatment of bipolar disorder. Adv Ther 2009 Jun; 26(6): 588–99
Swainston Harrison T, Goa KL. Long-acting risperidone: a review of its use in schizophrenia. CNS Drugs 2004; 18(2): 113–32
Fenton C, Scott LJ. Risperidone: a review of its use in the treatment of bipolar mania. CNS Drugs 2005; 19(5): 429–44
Risperdal® Consta® (risperidone) long-acting injection: US prescribing information. Titusville (NJ): Janssen, a division of Ortho-McNeil-Janssen Pharmaceuticals, Inc., 2010
Stahl SM. Describing an atypical antipsychotic: receptor binding and its role in pathophysiology. Prim Care Companion J Clin Psychiatry 2003; 5 Suppl. 3: 9–13
Richelson E. Receptor pharmacology of neuroleptics: relation to clinical effects. J Clin Psychiatry 1999; 60 Suppl. 10: 5–14
Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry 2006 Mar; 163(3): 396–401
Gefvert O, Eriksson B, Persson P, et al. Pharmacokinetics and D2 receptor occupancy of long-acting injectable risperidone (Risperdal Consta™) in patients with schizophrenia. Int J Neuropsychopharmacol 2005 Mar; 8(1): 27–36
Macfadden W, Alphs L, Haskins JT, et al. A randomized, double-blind, placebo-controlled study of maintenance treatment with adjunctive risperidone long-acting therapy in patients with bipolar I disorder who relapse frequently. Bipolar Disord 2009 Dec; 11(8): 827–39
Quiroz JA, Yatham LN, Palumbo JM, et al. Risperidone long-acting injectable monotherapy in the maintenance treatment of bipolar I disorder. Biol Psychiatry. Epub 2010 Mar 12
Baptista T, Kin NMKNY, Beaulieu S, et al. Obesity and related metabolic abnormalities during antipsychotic drug administration: mechanisms, management and research perspectives. Pharmacopsychiatry 2002 Nov; 35(6): 205–19
Eerdekens M, Van Hove I, Remmerie B, et al. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res 2004 Sep 1; 70(1): 91–100
Mannaert E, Vermeulen A, Remmerie B, et al. Pharmacokinetic profile of long-acting injectable risperidone at steady-state: comparison with oral administration. Encephale 2005 Sep–Oct; 31 (5 Pt 1): 609–15
Mannens G, Huang ML, Meuldermans W, et al. Absorption, metabolism, and excretion of risperidone in humans. Drug Metab Dispos 1993 Nov–Dec; 21(6): 1134–41
Yasui-Furukori N, Hidestrand M, Spina E, et al. Different enantioselective 9-hydroxylation of risperidone by the two human CYP2D6 and CYP3A4 enzymes. Drug Metab Dispos 2001 Oct; 29(10): 1263–8
Lasser RA, Ramstack JM, Grandolfi GP, et al. Long-acting injectable risperidone [Risperdal Consta™]: manufacture using Medisorb® microsphere technology, pharmacokinetics, and injection-site assessments [poster]. Annual Meeting of the American Psychiatric Association Institute on Psychiatric Services; 2002 Oct 9–13; Chicago (IL)
Thyssen A, Rusch S, Herben V, et al. Risperidone long-acting injection: pharmacokinetics following administration in deltoid versus gluteal muscle in schizophrenic patients. J Clin Pharmacol. Epub 2010 Jan 23
Turner N, Macfadden W, Turkoz I, et al. Assessment of cross-country differences in bipolar patients in the United States and India and the efficacy of risperidone long-acting injectable therapy [poster no. 40]. Institute on Psychiatric Services Annual Meeting; 2008 Oct 2–5; Chicago (IL)
Acknowledgements and Disclosures
This manuscript was reviewed by: P.B. Mitchell, School of Psychiatry, University of New South Wales, Sydney, New South Wales, Australia; R.M. Post, George Washington University, Washington, DC, USA; J.F. Rosenbaum, Department of Psychiatry, Harvard Medical School, Massachusetts General Hospital, Boston, Massachusetts, USA.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deeks, E.D. Risperidone Long-Acting Injection. Drugs 70, 1001–1012 (2010). https://doi.org/10.2165/11204480-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11204480-000000000-00000